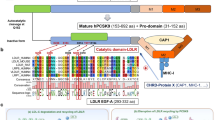
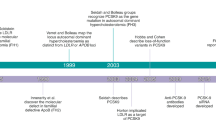
Abstract
The gene encoding PCSK9 was first identified and linked to the phenotype of familial hypercholesterolaemia approximately 15 years ago. Soon after, studies uncovered the role of PCSK9 in the regulation of LDL-receptor recycling and identified loss-of-function variants of PCSK9 that were associated with low circulating levels of LDL cholesterol (LDL-C) and a reduced risk of coronary artery disease. With amazing rapidity, monoclonal antibodies against PCSK9 were developed and studied in large clinical programmes. These PCSK9 inhibitors lowered plasma LDL-C levels by approximately 60%, even in patients already receiving maximum-dose statin therapy. In the past year, three cardiovascular outcome trials were completed and showed that PCSK9 inhibitors significantly reduce the risk of major vascular events. Reassuringly, this benefit comes with no major offsetting adverse events, such as an excess of myalgias, elevation of hepatic aminotransferases levels in the plasma, incident diabetes mellitus or neurocognitive adverse events. The clinical benefit of PCSK9 inhibitors seen in these trials occurred in the setting of reducing LDL-C levels to unprecedentedly low levels, suggesting that more aggressive LDL-C targets should be adopted. New technologies to inhibit PCSK9 are now being harnessed and might further revolutionize our treatment of dyslipidaemia.
Key points
-
Monoclonal antibodies targeting PCSK9 can lower plasma LDL-cholesterol (LDL-C) levels by approximately 60%.
-
In dedicated cardiovascular outcome trials, PCSK9 inhibitors significantly reduced the risk of major adverse cardiovascular events.
-
This benefit was consistent in patients with a baseline LDL-C level <70 mg/dl, who achieved an LDL-C level of approximately 20 mg/dl, which is well below the current guideline-recommended targets.
-
No offsetting safety concerns were reported in these trials over the timeframes studied.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Varret, M. et al. A third major locus for autosomal dominant hypercholesterolemia maps to 1p34.1-p32. Am. J. Hum. Genet. 64, 1378–1387 (1999).
Abifadel, M. et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat. Genet. 34, 154–156 (2003).
Horton, J. D., Cohen, J. C. & Hobbs, H. H. PCSK9: a convertase that coordinates LDL catabolism. J. Lipid Res. 50, S172–S177 (2009).
Maxwell, K. N. & Breslow, J. L. Adenoviral-mediated expression of Pcsk9 in mice results in a low-density lipoprotein receptor knockout phenotype. Proc. Natl Acad. Sci. USA 101, 7100–7105 (2004).
Maxwell, K. N., Fisher, E. A. & Breslow, J. L. Overexpression of PCSK9 accelerates the degradation of the LDLR in a post-endoplasmic reticulum compartment. Proc. Natl Acad. Sci. USA 102, 2069–2074 (2005).
Cohen, J. et al. Low LDL cholesterol in individuals of African descent resulting from frequent nonsense mutations in PCSK9. Nat. Genet. 37, 161–165 (2005).
Cohen, J. C. et al. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N. Engl. J. Med. 354, 1264–1272 (2006).
Kathiresan, S. & Myocardial Infarction Genetics, C. A. PCSK9 missense variant associated with a reduced risk of early-onset myocardial infarction. N. Engl. J. Med. 358, 2299–2300 (2008).
Ference, B. A. et al. Variation in PCSK9 and HMGCR and risk of cardiovascular disease and diabetes. N. Engl. J. Med. 375, 2144–2153 (2016).
Wasserman, S. et al. Comparison of LDL-C reduction using different evolocumab doses and intervals: biological insights and treatment implications. J. Cardiovasc. Pharmacol. Ther. 23, 423–432 (2018).
Koren, M. J. et al. Anti-PCSK9 monotherapy for hypercholesterolemia: the MENDEL-2 randomized, controlled phase III clinical trial of evolocumab. J. Am. Coll. Cardiol. 63, 2531–2540 (2014).
Robinson, J. G. et al. Effect of evolocumab or ezetimibe added to moderate- or high-intensity statin therapy on LDL-C lowering in patients with hypercholesterolemia: the LAPLACE-2 randomized clinical trial. JAMA 311, 1870–1882 (2014).
Stroes, E. et al. Anti-PCSK9 antibody effectively lowers cholesterol in patients with statin intolerance: the GAUSS-2 randomized, placebo-controlled phase 3 clinical trial of evolocumab. J. Am. Coll. Cardiol. 63, 2541–2548 (2014).
Nissen, S. E. et al. Efficacy and tolerability of evolocumab versus ezetimibe in patients with muscle-related statin intolerance: the GAUSS-3 randomized clinical trial. JAMA 315, 1580–1590 (2016).
Raal, F. J. et al. PCSK9 inhibition with evolocumab (AMG 145) in heterozygous familial hypercholesterolaemia (RUTHERFORD-2): a randomised, double-blind, placebo-controlled trial. Lancet 385, 331–340 (2015).
Stein, E. A. et al. Efficacy and safety of evolocumab (AMG 145), a fully human monoclonal antibody to PCSK9, in hyperlipidaemic patients on various background lipid therapies: pooled analysis of 1359 patients in four phase 2 trials. Eur. Heart J. 35, 2249–2259 (2014).
Desai, N. R. et al. Association between circulating baseline proprotein convertase subtilisin kexin type 9 levels and efficacy of evolocumab. JAMA Cardiol. 2, 556–560 (2017).
Raal, F. J. et al. Inhibition of PCSK9 with evolocumab in homozygous familial hypercholesterolaemia (TESLA part B): a randomised, double-blind, placebo-controlled trial. Lancet 385, 341–350 (2015).
Raal, F. J. et al. Long-term treatment with evolocumab added to conventional drug therapy, with or without apheresis, in patients with homozygous familial hypercholesterolaemia: an interim subset analysis of the open-label TAUSSIG study. Lancet Diabetes Endocrinol. 5, 280–290 (2017).
Roth, E. M. et al. Monotherapy with the PCSK9 inhibitor alirocumab versus ezetimibe in patients with hypercholesterolemia: results of a 24 week, double-blind, randomized phase 3 trial. Int. J. Cardiol. 176, 55–61 (2014).
Kereiakes, D. J. et al. Efficacy and safety of the proprotein convertase subtilisin/kexin type 9 inhibitor alirocumab among high cardiovascular risk patients on maximally tolerated statin therapy: the ODYSSEY COMBO I study. Am. Heart J. 169, 906–915.e13 (2015).
Cannon, C. P. et al. Efficacy and safety of alirocumab in high cardiovascular risk patients with inadequately controlled hypercholesterolaemia on maximally tolerated doses of statins: the ODYSSEY COMBO II randomized controlled trial. Eur. Heart J. 36, 1186–1194 (2015).
Moriarty, P. M. et al. Efficacy and safety of alirocumab versus ezetimibe in statin-intolerant patients, with a statin rechallenge arm: the ODYSSEY ALTERNATIVE randomized trial. J. Clin. Lipidol. 9, 758–769 (2015).
Robinson, J. G. et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N. Engl. J. Med. 372, 1489–1499 (2015).
Roth, E. M. et al. A phase III randomized trial evaluating alirocumab 300 mg every 4 weeks as monotherapy or add-on to statin: ODYSSEY CHOICE I. Atherosclerosis 254, 254–262 (2016).
Kastelein, J. J. et al. ODYSSEY FH I and FH II: 78 week results with alirocumab treatment in 735 patients with heterozygous familial hypercholesterolaemia. Eur. Heart J. 36, 2996–3003 (2015).
Ginsberg, H. N. et al. Efficacy and safety of alirocumab in patients with heterozygous familial hypercholesterolemia and LDL-C of 160 mg/dl or higher. Cardiovasc. Drugs Ther. 30, 473–483 (2016).
Hartgers, M. L. et al. Alirocumab efficacy in patients with double heterozygous, compound heterozygous, or homozygous familial hypercholesterolemia. J. Clin. Lipidol. 12, 390–396.e8 (2018).
US Department of Health & Human Services. Highlights of prescribing information: LIPITOR®. FDA.gov https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/020702s056lbl.pdf (2009).
Sabatine, M. S. et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N. Engl. J. Med. 376, 1713–1722 (2017).
Cannon, C. P. et al. Ezetimibe added to statin therapy after acute coronary syndromes. N. Engl. J. Med. 372, 2387–2397 (2015).
Desai, N. R. et al. AMG145, a monoclonal antibody against proprotein convertase subtilisin kexin type 9, significantly reduces lipoprotein(a) in hypercholesterolemic patients receiving statin therapy. Circulation 128, 962–969 (2013).
Bohula, E. A. et al. Inflammatory and cholesterol risk in the FOURIER trial (further cardiovascular outcomes research with PCSK9 inhibition in patients with elevated risk). Circulation 138, 131–140 (2018).
Sabatine, M. S. et al. Rationale and design of the Further cardiovascular OUtcomes Research with PCSK9 Inhibition in subjects with Elevated Risk trial. Am. Heart J. 173, 94–101 (2016).
Wiviott, S. D. The effects of adding the CETP inhibitor anacetrapib to statin on urgent and routine revascularization: results from the HPS 3/TIMI 55-REVEAL trial. J. Am. Coll. Cardiol. 71, A107 (2018).
Collins, R. et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet 388, 2532–2561 (2016).
Giugliano, R. P. et al. Clinical efficacy and safety of evolocumab in high-risk patients receiving a statin: secondary analysis of patients with low LDL cholesterol levels and in those already receiving a maximal-potency statin in a randomized clinical trial. JAMA Cardiol. 2, 1385–1391 (2017).
Ridker, P. M., Pradhan, A., MacFadyen, J. G., Libby, P. & Glynn, R. J. Cardiovascular benefits and diabetes risks of statin therapy in primary prevention: an analysis from the JUPITER trial. Lancet 380, 565–571 (2012).
Sabatine, M. S. et al. Cardiovascular safety and efficacy of the PCSK9 inhibitor evolocumab in patients with and without diabetes and the effect of evolocumab on glycaemia and risk of new-onset diabetes: a prespecified analysis of the FOURIER randomised controlled trial. Lancet Diabetes Endocrinol. 5, 941–950 (2017).
Giugliano, R. P. et al. Design and rationale of the EBBINGHAUS trial: a phase 3, double-blind, placebo-controlled, multicenter study to assess the effect of evolocumab on cognitive function in patients with clinically evident cardiovascular disease and receiving statin background lipid-lowering therapy — a cognitive study of patients enrolled in the FOURIER trial. Clin. Cardiol. 40, 59–65 (2017).
Giugliano, R. P., Sabatine, M. S. & Ott, B. R. Cognitive function in a randomized trial of evolocumab. N. Engl. J. Med. 377, 1997 (2017).
Ridker, P. M. et al. Lipid-reduction variability and antidrug-antibody formation with bococizumab. N. Engl. J. Med. 376, 1517–1526 (2017).
Ridker, P. M. et al. Evaluating bococizumab, a monoclonal antibody to PCSK9, on lipid levels and clinical events in broad patient groups with and without prior cardiovascular events: rationale and design of the studies of PCSK9 inhibition and the reduction of vascular events (SPIRE) lipid lowering and SPIRE cardiovascular outcomes trials. Am. Heart J. 178, 135–144 (2016).
Ridker, P. M. et al. Cardiovascular efficacy and safety of bococizumab in high-risk patients. N. Engl. J. Med. 376, 1527–1539 (2017).
Schwartz, G. G. et al. Effect of alirocumab, a monoclonal antibody to PCSK9, on long-term cardiovascular outcomes following acute coronary syndromes: rationale and design of the ODYSSEY outcomes trial. Am. Heart J. 168, 682–689 (2014).
Schwartz, G. et al. The ODYSSEY OUTCOMES trial: topline results — alirocumab in patients after acute coronary syndrome. Presented at the 67th Scientific Sessions of the American College of Cardiology (2018).
Wiviott, S. D. et al. A tale of two trials: a comparison of the post-acute coronary syndrome lipid-lowering trials A to Z and PROVE IT-TIMI 22. Circulation 113, 1406–1414 (2006).
Silverman, M. G. et al. Association between lowering LDL-C and cardiovascular risk reduction among different therapeutic interventions: a systematic review and meta-analysis. JAMA 316, 1289–1297 (2016).
Ference, B. A. et al. Reduction of low density lipoprotein-cholesterol and cardiovascular events with proprotein convertase subtilisin-kexin type 9 (PCSK9) inhibitors and statins: an analysis of FOURIER, SPIRE, and the Cholesterol Treatment Trialists Collaboration. Eur. Heart J. 39, 2540–2545 (2018).
Sacks, F. M. et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. N. Engl. J. Med. 335, 1001–1009 (1996).
Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N. Engl. J. Med. 339, 1349–1357 (1998).
Heart Protection Study Collaborative Group. MRC/BHF Heart Protection study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 360, 7–22 (2002).
LaRosa, J. C. et al. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N. Engl. J. Med. 352, 1425–1435 (2005).
Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 344, 1383–1389 (1994).
Hps Timi Reveal Collaborative Group, Bowman, L. et al. Effects of anacetrapib in patients with atherosclerotic vascular disease. N. Engl. J. Med. 377, 1217–1227 (2017).
Giugliano, R. P. et al. Clinical efficacy and safety of achieving very low LDL-cholesterol concentrations with the PCSK9 inhibitor evolocumab: a prespecified secondary analysis of the FOURIER trial. Lancet 390, 1962–1971 (2017).
Kazi, D. S. et al. Cost-effectiveness of PCSK9 inhibitor therapy in patients with heterozygous familial hypercholesterolemia or atherosclerotic cardiovascular disease. JAMA 316, 743–753 (2016).
Kazi, D. S. et al. Updated cost-effectiveness analysis of PCSK9 inhibitors based on the results of the FOURIER trial. JAMA 318, 748–750 (2017).
Fonarow, G. C. et al. Cost-effectiveness of evolocumab therapy for reducing cardiovascular events in patients with atherosclerotic cardiovascular disease. JAMA Cardiol. 2, 1069–1078 (2017).
Sabatine, M. S. & Giugliano, R. P. Low-density lipoprotein cholesterol treatment in the proprotein convertase subtilisin/kexin type 9 inhibitor era: getting back on target. JAMA Cardiol. 2, 935–936 (2017).
Navarese, E. P. et al. Association between baseline LDL-C level and total and cardiovascular mortality after LDL-C lowering: a systematic review and meta-analysis. JAMA 319, 1566–1579 (2018).
Sabatine, M. S., Wiviott, S. D., Im, K. A., Murphy, S. A. & Giugliano, R. P. Efficacy and safety of LDL-cholesterol lowering in patient populations starting with low LDL-cholesterol — a meta-analysis. JAMA Cardiol. 3, 823–828 (2018).
Bonaca, M. P., Braunwald, E. & Sabatine, M. S. Long-term use of ticagrelor in patients with prior myocardial infarction. N. Engl. J. Med. 373, 1274–1275 (2015).
Bonaca, M. P. et al. Efficacy and safety of ticagrelor over time in patients with prior MI in PEGASUS-TIMI 54. J. Am. Coll. Cardiol. 70, 1368–1375 (2017).
Dellborg, M. et al. Efficacy and safety with ticagrelor in patients with prior myocardial infarction in the approved European label: insights from PEGASUS-TIMI 54. Eur. Heart J. 38, P3670 (2017).
Bansilal, S. et al. Ticagrelor for secondary prevention of atherothrombotic events in patients with multivessel coronary disease. J. Am. Coll. Cardiol. 71, 489–496 (2018).
Sabatine, M. S. et al. Clinical benefit of evolocumab by severity and extent of coronary artery disease: an analysis from FOURIER. Circulation 138, 756–766 (2018).
Bonaca, M. P. et al. Low-density lipoprotein cholesterol lowering with evolocumab and outcomes in patients with peripheral artery disease: insights from the FOURIER trial (further cardiovascular outcomes research with PCSK9 inhibition in subjects with elevated risk). Circulation 137, 338–350 (2018).
Fitzgerald, K. et al. A highly durable RNAi therapeutic inhibitor of PCSK9. N. Engl. J. Med. 376, 41–51 (2017).
Ray, K. K. et al. Inclisiran in patients at high cardiovascular risk with elevated LDL cholesterol. N. Engl. J. Med. 376, 1430–1440 (2017).
TIMI Study Group. HPS-4/TIMI 65 ORION-4. TIMI.org http://www.timi.org/index.php?page=orion-4 (2018).
Landlinger, C. et al. The AT04A vaccine against proprotein convertase subtilisin/kexin type 9 reduces total cholesterol, vascular inflammation, and atherosclerosis in APOE*3Leiden.CETP mice. Eur. Heart J. 38, 2499–2507 (2017).
Pan, Y. et al. A therapeutic peptide vaccine against PCSK9. Sci. Rep. 7, 12534 (2017).
Wang, X. et al. CRISPR-Cas9 targeting of PCSK9 in human hepatocytes in vivo — brief report. Arterioscler. Thromb. Vasc. Biol. 36, 783–786 (2016).
Ding, Q. et al. Permanent alteration of PCSK9 with in vivo CRISPR-Cas9 genome editing. Circ. Res. 115, 488–492 (2014).
Thakore, P. I. et al. RNA-guided transcriptional silencing in vivo with S. aureus CRISPR-Cas9 repressors. Nat. Commun. 9, 1674 (2018).
Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170 000 participants in 26 randomised trials. Lancet 376, 1670–1681 (2010).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
M.S. received research grant support through Brigham and Women’s Hospital, Boston, USA, from Amgen, AstraZeneca, Daiichi-Sankyo, Eisai, GlaxoSmithKline, Intarcia, Janssen Research and Development, MedImmune, Merck, Novartis, Pfizer, Poxel, Takeda and The Medicines Company. M.S. also consulted for Amgen, AstraZeneca, Bristol-Myers Squibb, CVS Caremark, Esperion, Intarcia, Janssen Research and Development, MedImmune, Merck, Novartis and The Medicines Company.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sabatine, M.S. PCSK9 inhibitors: clinical evidence and implementation. Nat Rev Cardiol 16, 155–165 (2019). https://doi.org/10.1038/s41569-018-0107-8
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41569-018-0107-8