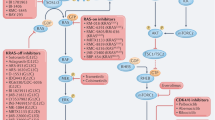
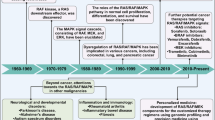
Abstract
The RAF family of kinases includes key activators of the pro-tumourigenic mitogen-activated protein kinase pathway. Hyperactivation of RAF proteins, particularly BRAF and CRAF, drives tumour progression and drug resistance in many types of cancer. Although BRAF is the most studied RAF protein, partially owing to its high mutation incidence in melanoma, the role of CRAF in tumourigenesis and drug resistance is becoming increasingly clinically relevant. Here, we summarize the main known regulatory mechanisms and gene alterations that contribute to CRAF activity, highlighting the different oncogenic roles of CRAF, and categorize RAF1 (CRAF) mutations according to the effect on kinase activity. Additionally, we emphasize the effect that CRAF alterations may have on drug resistance and how precision therapies could effectively target CRAF-dependent tumours. Here, we discuss preclinical and clinical findings that may lead to improved treatments for all types of oncogenic RAF1 alterations in cancer.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data that support the findings of this study are available at cBioportal and https://genie.cbioportal.org/.
References
Santarpia, L., Lippman, S. M. & El-Naggar, A. K. Targeting the MAPK–RAS–RAF signaling pathway in cancer therapy. Expert. Opin. Ther. Targets 16, 103–119 (2012).
Roberts, P. J. & Der, C. J. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene 26, 3291–3310 (2007).
Bollag, G. et al. Loss of NF1 results in activation of the Ras signaling pathway and leads to aberrant growth in haematopoietic cells. Nat. Genet. 12, 144–148 (1996).
Freeman, A. K., Ritt, D. A. & Morrison, D. K. Effects of Raf dimerization and its inhibition on normal and disease-associated Raf signaling. Mol. Cell 49, 751–758 (2013).
Kong, T. et al. Role of the extracellular signal-regulated kinase 1/2 signaling pathway in ischemia-reperfusion injury. Front. Physiol. 10, 1038 (2019).
Sawai, H. et al. Activation of focal adhesion kinase enhances the adhesion and invasion of pancreatic cancer cells via extracellular signal-regulated kinase-1/2 signaling pathway activation. Mol. Cancer 4, 37 (2005).
Tartaglia, M., Gelb, B. D. & Zenker, M. Noonan syndrome and clinically related disorders. Best. Pract. Res. Clin. Endocrinol. Metab. 25, 161–179 (2011).
Wortzel, I. & Seger, R. The ERK cascade: distinct functions within various subcellular organelles. Genes. Cancer 2, 195–209 (2011).
Guo, Y. J. et al. ERK/MAPK signalling pathway and tumorigenesis. Exp. Ther. Med. 19, 1997–2007 (2020).
Davies, H. et al. Mutations of the BRAF gene in human cancer. Nature 417, 949–954 (2002).
Yuan, J., Dong, X., Yap, J. & Hu, J. The MAPK and AMPK signalings: interplay and implication in targeted cancer therapy. J. Hematol. Oncol. 13, 113 (2020).
Tidyman, W. E. & Rauen, K. A. Pathogenetics of the RASopathies. Hum. Mol. Genet. 25, R123–R132 (2016).
Dankner, M., Rose, A. A. N., Rajkumar, S., Siegel, P. M. & Watson, I. R. Classifying BRAF alterations in cancer: new rational therapeutic strategies for actionable mutations. Oncogene 37, 3183–3199 (2018).
Davis, E. J., Johnson, D. B., Sosman, J. A. & Chandra, S. Melanoma: what do all the mutations mean? Cancer 124, 3490–3499 (2018).
Johnson, D. B., Nebhan, C. A. & Noel, M. S. MEK inhibitors in non-V600 BRAF mutations and fusions. Oncotarget 11, 3900–3903 (2020).
Subbiah, V., Baik, C. & Kirkwood, J. M. Clinical development of BRAF plus MEK inhibitor combinations. Trends Cancer 6, 797–810 (2020).
Bracht, J. W. et al. BRAF mutations classes I, II, and III in NSCLC patients included in the SLLIP trial: the need for a new pre-clinical treatment rationale. Cancers 11, 1381 (2019).
Yap, J. L., Worlikar, S., MacKerell, A. D. Jr, Shapiro, P. & Fletcher, S. Small-molecule inhibitors of the ERK signaling pathway: towards novel anticancer therapeutics. ChemMedChem 6, 38–48 (2011).
Skoulidis, F. et al. Sotorasib for lung cancers with KRAS p.G12C mutation. N. Engl. J. Med. 384, 2371–2381 (2021).
Sumimoto, H., Imabayashi, F., Iwata, T. & Kawakami, Y. The BRAF-MAPK signaling pathway is essential for cancer-immune evasion in human melanoma cells. J. Exp. Med. 203, 1651–1656 (2006).
Drosten, M. & Barbacid, M. Targeting the MAPK pathway in KRAS-driven tumors. Cancer Cell 37, 543–550 (2020).
Hymowitz, S. G. & Malek, S. Targeting the MAPK pathway in RAS mutant cancers. Cold Spring Harb. Perspect. Med. 8, a031492 (2018).
Bekele, R. T. et al. RAF1 amplification drives a subset of bladder tumors and confers sensitivity to MAPK-directed therapeutics. J. Clin. Invest. 131, e147849 (2021). This is the first study, to our knowledge, to report that tumours with RAF1 amplification could be growth inhibited in vivo with novel pan-RAF inhibitors.
Heidorn, S. J. et al. Kinase-dead BRAF and oncogenic RAS cooperate to drive tumor progression through CRAF. Cell 140, 209–221 (2010).
Montagut, C. et al. Elevated CRAF as a potential mechanism of acquired resistance to BRAF inhibition in melanoma. Cancer Res. 68, 4853–4861 (2008).
Dankner, M. et al. Dual MAPK inhibition is an effective therapeutic strategy for a subset of class II BRAF mutant melanomas. Clin. Cancer Res. 24, 6483–6494 (2018).
Storm, S. M., Cleveland, J. L. & Rapp, U. R. Expression of raf family proto-oncogenes in normal mouse tissues. Oncogene 5, 345–351 (1990).
Tran, T. H. et al. KRAS interaction with RAF1 RAS-binding domain and cysteine-rich domain provides insights into RAS-mediated RAF activation. Nat. Commun. 12, 1176 (2021).
Vojtek, A. B., Hollenberg, S. M. & Cooper, J. A. Mammalian Ras interacts directly with the serine/threonine kinase Raf. Cell 74, 205–214 (1993).
Warne, P. H., Viciana, P. R. & Downward, J. Direct interaction of Ras and the amino-terminal region of Raf-1 in vitro. Nature 364, 352–355 (1993).
Kubicek, M. et al. Dephosphorylation of Ser-259 regulates Raf-1 membrane association. J. Biol. Chem. 277, 7913–7919 (2002).
Huang, W., Alessandrini, A., Crews, C. M. & Erikson, R. L. Raf-1 forms a stable complex with Mek1 and activates Mek1 by serine phosphorylation. Proc. Natl Acad. Sci. USA 90, 10947–10951 (1993).
Fantl, W. J. et al. Activation of Raf-1 by 14-3-3 proteins. Nature 371, 612–614 (1994).
Fu, H. et al. Interaction of the protein kinase Raf-1 with 14-3-3 proteins. Science 266, 126–129 (1994).
Yip-Schneider, M. T. et al. Regulation of the Raf-1 kinase domain by phosphorylation and 14-3-3 association. Biochem. J. 351, 151–159, (2000).
Chong, H., Vikis, H. G. & Guan, K. L. Mechanisms of regulating the Raf kinase family. Cell Signal. 15, 463–469 (2003).
Dhillon, A. S. & Kolch, W. Untying the regulation of the Raf-1 kinase. Arch. Biochem. Biophys. 404, 3–9 (2002).
Chong, H. & Guan, K. L. Regulation of Raf through phosphorylation and N terminus-C terminus interaction. J. Biol. Chem. 278, 36269–36276 (2003).
Tran, N. H., Wu, X. & Frost, J. A. B-Raf and Raf-1 are regulated by distinct autoregulatory mechanisms. J. Biol. Chem. 280, 16244–16253 (2005).
Cutler, R. E. Jr, Stephens, R. M., Saracino, M. R. & Morrison, D. K. Autoregulation of the Raf-1 serine/threonine kinase. Proc. Natl Acad. Sci. USA 95, 9214–9219 (1998).
Rommel, C. et al. Activated Ras displaces 14-3-3 protein from the amino terminus of c-Raf-1. Oncogene 12, 609–619 (1996).
Tzivion, G., Luo, Z. & Avruch, J. A dimeric 14-3-3 protein is an essential cofactor for Raf kinase activity. Nature 394, 88–92 (1998).
Dumaz, N. & Marais, R. Protein kinase A blocks Raf-1 activity by stimulating 14-3-3 binding and blocking Raf-1 interaction with Ras. J. Biol. Chem. 278, 29819–29823 (2003).
Stokoe, D., Macdonald, S. G., Cadwallader, K., Symons, M. & Hancock, J. F. Activation of Raf as a result of recruitment to the plasma membrane. Science 264, 1463–1467 (1994).
Leevers, S. J., Paterson, H. F. & Marshall, C. J. Requirement for Ras in Raf activation is overcome by targeting Raf to the plasma membrane. Nature 369, 411–414 (1994).
Fabian, J. R., Vojtek, A. B., Cooper, J. A. & Morrison, D. K. A single amino acid change in Raf-1 inhibits Ras binding and alters Raf-1 function. Proc. Natl Acad. Sci. 91, 5982–5986 (1994).
Fang, Z. et al. Multivalent assembly of KRAS with the RAS-binding and cysteine-rich domains of CRAF on the membrane. Proc. Natl Acad. Sci. USA 117, 12101–12108 (2020).
Travers, T. et al. Molecular recognition of RAS/RAF complex at the membrane: role of RAF cysteine-rich domain. Sci. Rep. 8, 8461 (2018).
Dhillon, A. S. et al. Cyclic AMP-dependent kinase regulates Raf-1 kinase mainly by phosphorylation of serine 259. Mol. Cell Biol. 22, 3237–3246 (2002).
Rodriguez-Viciana, P., Oses-Prieto, J., Burlingame, A., Fried, M. & McCormick, F. A phosphatase holoenzyme comprised of Shoc2/Sur8 and the catalytic subunit of PP1 functions as an M-Ras effector to modulate Raf activity. Mol. Cell 22, 217–230 (2006).
Young, LucyC. et al. An MRAS, SHOC2, and SCRIB complex coordinates ERK pathway activation with polarity and tumorigenic growth. Mol. Cell 52, 679–692 (2013).
Kwon, J. J. et al. Structure–function analysis of the SHOC2–MRAS–PP1C holophosphatase complex. Nature 609, 408–415 (2022).
Hauseman, Z. J. et al. Structure of the MRAS–SHOC2–PP1C phosphatase complex. Nature 609, 416–423 (2022).
Liau, N. P. D. et al. Structural basis for SHOC2 modulation of RAS signalling. Nature 609, 400–407 (2022).
Dhillon, A. S., Meikle, S., Yazici, Z., Eulitz, M. & Kolch, W. Regulation of Raf-1 activation and signalling by dephosphorylation. EMBO J. 21, 64–71 (2002). This study demonstrates that the dephosphorylation of Ser259 is an essential step in CRAF activation and that the RAF1 S259A mutation prevented 14-3-3 binding.
Fabian, J. R., Daar, I. O. & Morrison, D. K. Critical tyrosine residues regulate the enzymatic and biological activity of Raf-1 kinase. Mol. Cell Biol. 13, 7170–7179 (1993).
King, A. J. et al. The protein kinase Pak3 positively regulates Raf-1 activity through phosphorylation of serine 338. Nature 396, 180–183 (1998).
Chong, H., Lee, J. & Guan, K.-L. Positive and negative regulation of Raf kinase activity and function by phosphorylation. EMBO J. 20, 3716–3727 (2001).
Chiloeches, A., Mason, C. S. & Marais, R. S338 phosphorylation of Raf-1 is independent of phosphatidylinositol 3-kinase and Pak3. Mol. Cell Biol. 21, 2423–2434 (2001).
Emuss, V., Garnett, M., Mason, C. & Marais, R. Mutations of C-RAF are rare in human cancer because C-RAF has a low basal kinase activity compared with B-RAF. Cancer Res. 65, 9719–9726 (2005).
Zang, M. et al. Characterization of Ser338 phosphorylation for Raf-1 activation. J. Biol. Chem. 283, 31429–31437 (2008).
Marais, R., Light, Y., Paterson, H. F., Mason, C. S. & Marshall, C. J. Differential regulation of Raf-1, A-Raf, and B-Raf by oncogenic ras and tyrosine kinases. J. Biol. Chem. 272, 4378–4383 (1997).
Farrar, M. A., Alberol-Ila, J. & Perlmutter, R. M. Activation of the Raf-1 kinase cascade by coumermycin-induced dimerization. Nature 383, 178–181 (1996).
Luo, Z. et al. Oligomerization activates c-Raf-1 through a Ras-dependent mechanism. Nature 383, 181–185 (1996).
Weber, C. K., Slupsky, J. R., Kalmes, H. A. & Rapp, U. R. Active Ras induces heterodimerization of cRaf and BRaf. Cancer Res. 61, 3595–3598 (2001).
Rushworth, L. K., Hindley, A. D., O’Neill, E. & Kolch, W. Regulation and role of Raf-1/B-Raf heterodimerization. Mol. Cell. Biol. 26, 2262–2272 (2006).
Garnett, M. J., Rana, S., Paterson, H., Barford, D. & Marais, R. Wild-type and mutant B-RAF activate C-RAF through distinct mechanisms involving heterodimerization. Mol. Cell 20, 963–969 (2005).
Dougherty, M. K. et al. Regulation of Raf-1 by direct feedback phosphorylation. Mol. Cell 17, 215–224 (2005).
Ritt, D. A., Monson, D. M., Specht, S. I. & Morrison, D. K. Impact of feedback phosphorylation and Raf heterodimerization on normal and mutant B-Raf signaling. Mol. Cell Biol. 30, 806–819 (2010).
Noble, C. et al. CRAF autophosphorylation of serine 621 is required to prevent its proteasome-mediated degradation. Mol. Cell 31, 862–872 (2008).
Mitra, S., Ghosh, B., Gayen, N., Roy, J. & Mandal, A. K. Bipartite role of heat shock protein 90 (Hsp90) keeps CRAF kinase poised for activation. J. Biol. Chem. 291, 24579–24593 (2016).
García-Alonso, S. et al. Structure of the RAF1-HSP90-CDC37 complex reveals the basis of RAF1 regulation. Mol. Cell 82, 3438–3452.e8 (2022). This study reports the structure of the CRAF protein in complex with HSP90 and CDC37 and shows how CRAF interacts with 14-3-3 dimers.
Feng, D., Sheng-Dong, L., Tong, W. & Zhen-Xian, D. O-GlcNAcylation of RAF1 increases its stabilization and induces the renal fibrosis. Biochim. Biophys. Acta Mol. Basis Dis. 1866, 165556 (2020).
Chan, L. H. et al. PRMT6 regulates RAS/RAF binding and MEK/ERK-mediated cancer stemness activities in hepatocellular carcinoma through CRAF methylation. Cell Rep. 25, 690–701.e8 (2018).
Andreu-Pérez, P. et al. Protein arginine methyltransferase 5 regulates ERK1/2 signal transduction amplitude and cell fate through CRAF. Sci. Signal. 4, ra58 (2011).
Yeung, K. et al. Mechanism of suppression of the Raf/MEK/extracellular signal-regulated kinase pathway by the Raf kinase inhibitor protein. Mol. Cell. Biol. 20, 3079–3085 (2000).
Yao, B. et al. Phosphorylation of Raf by ceramide-activated protein kinase. Nature 378, 307–310 (1995).
Zhu, J. et al. Identification of Raf-1 S471 as a novel phosphorylation site critical for Raf-1 and B-Raf kinase activities and for MEK binding. Mol. Biol. Cell 16, 4733–4744 (2005).
Ravi, R. K. et al. Activated Raf-1 causes growth arrest in human small cell lung cancer cells. J. Clin. Invest. 101, 153–159 (1998).
Imielinski, M. et al. Oncogenic and sorafenib-sensitive ARAF mutations in lung adenocarcinoma. J. Clin. Invest. 124, 1582–1586 (2014).
Noeparast, A. et al. CRAF mutations in lung cancer can be oncogenic and predict sensitivity to combined type II RAF and MEK inhibition. Oncogene 38, 5933–5941 (2019).
Atefi, M. et al. CRAF R391W is a melanoma driver oncogene. Sci. Rep. 6, 27454 (2016).
Antony, R., Emery, C. M., Sawyer, A. M. & Garraway, L. A. C-RAF mutations confer resistance to RAF inhibitors. Cancer Res. 73, 4840–4851 (2013).
Hodis, E. et al. A landscape of driver mutations in melanoma. Cell 150, 251–263 (2012).
Wu, X. et al. Increased BRAF heterodimerization is the common pathogenic mechanism for noonan syndrome-associated RAF1 mutants. Mol. Cell Biol. 32, 3872–3890 (2012).
Hüser, M. et al. MEK kinase activity is not necessary for Raf-1 function. EMBO J. 20, 1940–1951 (2001).
Mikula, M. et al. Embryonic lethality and fetal liver apoptosis in mice lacking the c-raf-1 gene. EMBO J. 20, 1952–1962 (2001).
Wojnowski, L. et al. Craf-1 protein kinase is essential for mouse development. Mech. Dev. 76, 141–149 (1998).
Jin, S., Zhuo, Y., Guo, W. & Field, J. p21-activated Kinase 1 (Pak1)-dependent phosphorylation of Raf-1 regulates its mitochondrial localization, phosphorylation of BAD, and Bcl-2 association. J. Biol. Chem. 280, 24698–24705 (2005).
Wang, H. G., Rapp, U. R. & Reed, J. C. Bcl-2 targets the protein kinase Raf-1 to mitochondria. Cell 87, 629–638 (1996).
Gollob, J. A., Wilhelm, S., Carter, C. & Kelley, S. L. Role of Raf kinase in cancer: therapeutic potential of targeting the Raf/MEK/ERK signal transduction pathway. Semin. Oncol. 33, 392–406 (2006).
Smalley, K. S. et al. CRAF inhibition induces apoptosis in melanoma cells with non-V600E BRAF mutations. Oncogene 28, 85–94 (2009).
O’Neill, E., Rushworth, L., Baccarini, M. & Kolch, W. Role of the kinase MST2 in suppression of apoptosis by the proto-oncogene product Raf-1. Science 306, 2267–2270 (2004).
Chen, J., Fujii, K., Zhang, L., Roberts, T. & Fu, H. Raf-1 promotes cell survival by antagonizing apoptosis signal-regulating kinase 1 through a MEK-ERK independent mechanism. Proc. Natl Acad. Sci. USA 98, 7783–7788 (2001).
Dasgupta, P. et al. Nicotine induces cell proliferation by beta-arrestin-mediated activation of Src and Rb-Raf-1 pathways. J. Clin. Invest. 116, 2208–2217 (2006).
Kerkhoff, E. & Rapp, U. R. Induction of cell proliferation in quiescent NIH 3T3 cells by oncogenic c-Raf-1. Mol. Cell Biol. 17, 2576–2586 (1997).
Wang, S., Ghosh, R. N. & Chellappan, S. P. Raf-1 physically interacts with Rb and regulates its function: a link between mitogenic signaling and cell cycle regulation. Mol. Cell. Biol. 18, 7487–7498 (1998).
Mielgo, A. et al. A MEK-independent role for CRAF in mitosis and tumor progression. Nat. Med. 17, 1641–1645 (2011).
Simanshu, D. K., Nissley, D. V. & McCormick, F. RAS proteins and their regulators in human disease. Cell 170, 17–33 (2017).
Ehrenreiter, K. et al. Raf-1 addiction in Ras-induced skin carcinogenesis. Cancer Cell 16, 149–160 (2009).
Sanclemente, M. et al. c-RAF ablation induces regression of advanced Kras/Trp53 mutant lung adenocarcinomas by a mechanism independent of MAPK signaling. Cancer Cell 33, 217–228.e4 (2018).
Blasco, R. B. et al. c-Raf, but not B-Raf, is essential for development of K-Ras oncogene-driven non-small cell lung carcinoma. Cancer Cell 19, 652–663 (2011).
Karreth, F. A., Frese, K. K., DeNicola, G. M., Baccarini, M. & Tuveson, D. A. C-Raf is required for the initiation of lung cancer by K-Ras(G12D). Cancer Discov. 1, 128–136 (2011).
Venkatanarayan, A. et al. CRAF dimerization with ARAF regulates KRAS-driven tumor growth. Cell Rep. 38, 110351 (2022). This study reports that the kinase function of CRAF was dispensible but that the dimerization function of CRAF is required for the proliferation of KRAS mutant cancers.
Dumaz, N. et al. In melanoma, RAS mutations are accompanied by switching signaling from BRAF to CRAF and disrupted cyclic AMP signaling. Cancer Res. 66, 9483–9491 (2006).
Yan, J., Roy, S., Apolloni, A., Lane, A. & Hancock, J. F. Ras isoforms vary in their ability to activate Raf-1 and phosphoinositide 3-kinase. J. Biol. Chem. 273, 24052–24056 (1998).
Shu, L., Wang, D., Saba, N. F. & Chen, Z. G. A historic perspective and overview of H-Ras structure, oncogenicity, and targeting. Mol. Cancer Ther. 19, 999–1007 (2020).
Suire, S., Hawkins, P. & Stephens, L. Activation of phosphoinositide 3-kinase gamma by Ras. Curr. Biol. 12, 1068–1075 (2002).
Webb, C. P., Van Aelst, L., Wigler, M. H. & Vande Woude, G. F. Signaling pathways in Ras-mediated tumorigenicity and metastasis. Proc. Natl Acad. Sci. USA 95, 8773–8778 (1998).
Dorard, C. et al. RAF1 contributes to cell proliferation and STAT3 activation in colorectal cancer independently of microsatellite and KRAS status. Oncogene 42, 1649–1660 (2023).
Blasco, M. T. et al. Complete regression of advanced pancreatic ductal adenocarcinomas upon combined inhibition of EGFR and C-RAF. Cancer Cell 35, 573–587.e6 (2019).
Oka, H. et al. Constitutive activation of mitogen-activated protein (MAP) kinases in human renal cell carcinoma. Cancer Res. 55, 4182–4187 (1995).
Hwang, Y. H. et al. Over-expression of c-raf-1 proto-oncogene in liver cirrhosis and hepatocellular carcinoma. Hepatol. Res. 29, 113–121 (2004).
Wilhelm, S. M. et al. Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling. Mol. Cancer Ther. 7, 3129–3140 (2008).
Esteban-Burgos, L. et al. Tumor regression and resistance mechanisms upon CDK4 and RAF1 inactivation in KRAS/P53 mutant lung adenocarcinomas. Proc. Natl Acad. Sci. USA 117, 24415–24426 (2020).
Moon, A., Park, J. Y., Sung, J. Y., Park, Y. K. & Kim, Y. W. Reduced expression of Raf-1 kinase inhibitory protein in renal cell carcinoma: a significant prognostic marker. Pathology 44, 534–539 (2012).
Yao, Z. et al. BRAF mutants evade ERK-dependent feedback by different mechanisms that determine their sensitivity to pharmacologic inhibition. Cancer Cell 28, 370–383 (2015).
Chen, S. H. et al. Oncogenic BRAF deletions that function as homodimers and are sensitive to inhibition by RAF dimer inhibitor LY3009120. Cancer Discov. 6, 300–315 (2016).
Yao, Z. et al. Tumours with class 3 BRAF mutants are sensitive to the inhibition of activated RAS. Nature 548, 234–238 (2017).
Dankner, M. et al. Clinical activity of mitogen-activated protein kinase-targeted therapies in patients with non-V600 BRAF-mutant tumors. JCO Precis. Oncol. 6, e2200107 (2022).
Simon, R. et al. High-throughput tissue microarray analysis of 3p25 (RAF1) and 8p12 (FGFR1) copy number alterations in urinary bladder cancer. Cancer Res. 61, 4514–4519 (2001).
Mukherjee, R., Bartlett, J. M. S., Krishna, N. S., Underwood, M. A. & Edwards, J. Raf-1 expression may influence progression to androgen insensitive prostate cancer. Prostate 64, 101–107 (2005).
Edwards, J., Krishna, N. S., Witton, C. J. & Bartlett, J. M. Gene amplifications associated with the development of hormone-resistant prostate cancer. Clin. Cancer Res. 9, 5271–5281 (2003).
Wilson, M. A. et al. Copy number changes are associated with response to treatment with carboplatin, paclitaxel, and sorafenib in melanoma. Clin. Cancer Res. 22, 374–382 (2016).
Stransky, N., Cerami, E., Schalm, S., Kim, J. L. & Lengauer, C. The landscape of kinase fusions in cancer. Nat. Commun. 5, 4846 (2014).
Ren, G. et al. Identification of frequent BRAF copy number gain and alterations of RAF genes in Chinese prostate cancer. Genes. Chromosomes Cancer 51, 1014–1023 (2012).
Palanisamy, N. et al. Rearrangements of the RAF kinase pathway in prostate cancer, gastric cancer and melanoma. Nat. Med. 16, 793–798 (2010).
Baltres, A. et al. Malignant melanoma with areas of rhabdomyosarcomatous differentiation arising in a giant congenital nevus with RAF1 gene fusion. Pigment. Cell Melanoma Res. 32, 708–713 (2019).
Williams, E. A. et al. Melanomas with activating RAF1 fusions: clinical, histopathologic, and molecular profiles. Mod. Pathol. 33, 1466–1474 (2020).
LeBlanc, R. E., Lefferts, J. A., Baker, M. L. & Linos, K. D. Novel LRRFIP2-RAF1 fusion identified in an acral melanoma: a review of the literature on melanocytic proliferations with RAF1 fusions and the potential therapeutic implications. J. Cutan. Pathol. 47, 1181–1186 (2020).
Donati, M. et al. RAF1 gene fusions as a possible driver mechanism in rare BAP1-inactivated melanocytic tumors: a report of 2 cases. Am. J. Dermatopathol. 42, 961–966 (2020).
Pacaud, A., Amintas, S., Boussemart, L., Cappellen, D. & Gérard, E. A case of multi-metastatic melanoma with RAF1 fusion: a surprising response to anti-MEK therapy. Eur. J. Cancer 147, 161–163 (2021).
McEvoy, C. R. et al. Profound MEK inhibitor response in a cutaneous melanoma harboring a GOLGA4-RAF1 fusion. J. Clin. Invest. 129, 1940–1945 (2019).
Prall, O. W. J. et al. RAF1 rearrangements are common in pancreatic acinar cell carcinomas. Mod. Pathol. 33, 1811–1821 (2020).
Hendifar, A. et al. Retrospective case series analysis of RAF family alterations in pancreatic cancer: real-world outcomes from targeted and standard therapies. JCO Precis Oncol. 5, PO.20.00494 (2021).
Chmielecki, J. et al. Comprehensive genomic profiling of pancreatic acinar cell carcinomas identifies recurrent RAF fusions and frequent inactivation of DNA repair genes. Cancer Discov. 4, 1398–1405 (2014).
Daoud, E. V. et al. Spinal pleomorphic xanthoastrocytoma with a QKI-RAF1 fusion. J. Neuropathol. Exp. Neurol. 78, 10–14 (2019).
Phillips, J. J. et al. The genetic landscape of anaplastic pleomorphic xanthoastrocytoma. Brain Pathol. 29, 85–96 (2019).
Phillips, J. J. et al. Activating NRF1-BRAF and ATG7-RAF1 fusions in anaplastic pleomorphic xanthoastrocytoma without BRAF p.V600E mutation. Acta Neuropathol. 132, 757–760 (2016).
Shaw, A. T., Hsu, P. P., Awad, M. M. & Engelman, J. A. Tyrosine kinase gene rearrangements in epithelial malignancies. Nat. Rev. Cancer 13, 772–787 (2013).
Hicks, J. K., Henderson-Jackson, E., Duggan, J., Joyce, D. M. & Brohl, A. S. Identification of a novel MTAP-RAF1 fusion in a soft tissue sarcoma. Diagn. Pathol. 13, 77 (2018).
Mok, Y. et al. Spindle cell tumour with S100 and CD34 co-expression showing PDZRN3-RAF1 rearrangement — a recently described entity. Histopathology 74, 1109–1111 (2019).
Suurmeijer, A. J. H. et al. A novel group of spindle cell tumors defined by S100 and CD34 co-expression shows recurrent fusions involving RAF1, BRAF, and NTRK1/2 genes. Genes. Chromosomes Cancer 57, 611–621 (2018).
Necchi, A. et al. Comprehensive genomic profiling of upper-tract and bladder urothelial carcinoma. Eur. Urol. Focus. 7, 1339–1346 (2021).
Liu, H. et al. Identifying and targeting sporadic oncogenic genetic aberrations in mouse models of triple-negative breast cancer. Cancer Discov. 8, 354–369 (2018).
Jain, P. et al. CRAF gene fusions in pediatric low-grade gliomas define a distinct drug response based on dimerization profiles. Oncogene 36, 6348–6358 (2017).
Jones, D. T. et al. Oncogenic RAF1 rearrangement and a novel BRAF mutation as alternatives to KIAA1549:BRAF fusion in activating the MAPK pathway in pilocytic astrocytoma. Oncogene 28, 2119–2123 (2009).
Shah, K. et al. Androgen receptor signaling regulates the transcriptome of prostate cancer cells by modulating global alternative splicing. Oncogene 39, 6172–6189 (2020).
McFarlin, D. R. & Gould, M. N. Rat mammary carcinogenesis induced by in situ expression of constitutive Raf kinase activity is prevented by tethering Raf to the plasma membrane. Carcinogenesis 24, 1149–1153 (2003).
Fukui, M., Yamamoto, T., Kawai, S., Mitsunobu, F. & Toyoshima, K. Molecular cloning and characterization of an activated human c-raf-1 gene. Mol. Cell. Biol. 7, 1776–1781 (1987).
Ishikawa, F., Takaku, F., Hayashi, K., Nagao, M. & Sugimura, T. Activation of rat c-raf during transfection of hepatocellular carcinoma DNA. Proc. Natl Acad. Sci. 83, 3209–3212 (1986).
Mölders, H. et al. Integration of transfected LTR sequences into the c-raf proto-oncogene: activation by promoter insertion. EMBO J. 4, 693–698 (1985).
Stanton, V. P. & Cooper, G. M. Activation of human raf transforming genes by deletion of normal amino-terminal coding sequences. Mol. Cell. Biol. 7, 1171–1179 (1987).
Turner, J. A. et al. BRAF fusions identified in melanomas have variable treatment responses and phenotypes. Oncogene 38, 1296–1308 (2019).
Yde, C. W. et al. A new NFIA:RAF1 fusion activating the MAPK pathway in pilocytic astrocytoma. Cancer Genet. 209, 440–444 (2016).
Gao, J. et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal. 6, pl1 (2013).
Kobayashi, T. et al. Molecular and clinical analysis of RAF1 in Noonan syndrome and related disorders: dephosphorylation of serine 259 as the essential mechanism for mutant activation. Hum. Mutat. 31, 284–294 (2010).
Chan, E. Y. W., Stang, S. L., Bottorff, D. A. & Stone, J. C. Mutations in conserved regions 1, 2, and 3 of Raf-1 that activate transforming activity. Mol. Carcinog. 33, 189–197 (2002). This study demonstrates that the RAF1 S259A and G361S mutations are capable of inducing cellular transformation in vitro.
Stanton, V. P. Jr, Nichols, D. W., Laudano, A. P. & Cooper, G. M. Definition of the human raf amino-terminal regulatory region by deletion mutagenesis. Mol. Cell Biol. 9, 639–647 (1989).
Ishikawa, F. et al. Identification of a transforming activity suppressing sequence in the c-raf oncogene. Oncogene 3, 653–658 (1988).
Heidecker, G. et al. Mutational activation of c-raf-1 and definition of the minimal transforming sequence. Mol. Cell Biol. 10, 2503–2512 (1990).
Bruder, J. T., Heidecker, G. & Rapp, U. R. Serum-, TPA-, and Ras-induced expression from Ap-1/Ets-driven promoters requires Raf-1 kinase. Genes. Dev. 6, 545–556 (1992).
Michaud, N. R., Fabian, J. R., Mathes, K. D. & Morrison, D. K. 14-3-3 is not essential for Raf-1 function: identification of Raf-1 proteins that are biologically activated in a 14-3-3- and Ras-independent manner. Mol. Cell Biol. 15, 3390–3397 (1995).
Zhao, Q., Boschelli, F., Caplan, A. J. & Arndt, K. T. Identification of a conserved sequence motif that promotes Cdc37 and cyclin D1 binding to Cdk4. J. Biol. Chem. 279, 12560–12564 (2004).
Zebisch, A. et al. Two transforming C-RAF germ-line mutations identified in patients with therapy-related acute myeloid leukemia. Cancer Res. 66, 3401–3408 (2006).
Diamond, E. L. et al. Efficacy of MEK inhibition in patients with histiocytic neoplasms. Nature 567, 521–524 (2019).
The, A. P. G. C. et al. AACR project GENIE: powering precision medicine through an International Consortium. Cancer Discov. 7, 818–831 (2017).
Pandit, B. et al. Gain-of-function RAF1 mutations cause Noonan and LEOPARD syndromes with hypertrophic cardiomyopathy. Nat. Genet. 39, 1007–1012 (2007).
Molzan, M. et al. Impaired binding of 14-3-3 to C-RAF in Noonan syndrome suggests new approaches in diseases with increased Ras signaling. Mol. Cell Biol. 30, 4698–4711 (2010).
Razzaque, M. A. et al. Germline gain-of-function mutations in RAF1 cause Noonan syndrome. Nat. Genet. 39, 1013–1017 (2007).
Proietti, I. et al. Mechanisms of acquired BRAF inhibitor resistance in melanoma: a systematic review. Cancers 12, 2801 (2020).
Petti, C. et al. Truncated RAF kinases drive resistance to MET inhibition in MET-addicted cancer cells. Oncotarget 6, 221–233 (2015).
Hong, A. et al. Durable suppression of acquired MEK inhibitor resistance in cancer by sequestering MEK from ERK and promoting antitumor T-cell immunity. Cancer Discov. 11, 714–735 (2021). This study demonstrates for the first time, to our knowledge, that pan-RAF inhibitors used alone or in combination with MEK inhibitor can modify the tumour immune microenvironment in preclinical models of melanoma.
Song, K. et al. Plasticity of extrachromosomal and intrachromosomal BRAF amplifications in overcoming targeted therapy dosage challenges. Cancer Discov. 12, 1046–1069 (2022).
Awad, M. M. et al. Acquired resistance to KRASG12C inhibition in cancer. N. Engl. J. Med. 384, 2382–2393 (2021). This is the first report, to our knowledge, of two acquired RAF1 fusion proteins occurring in a patient with acquired resistance to the KRAS G12C inhibitor, adagrasib.
Zhao, Y. et al. Diverse alterations associated with resistance to KRAS(G12C) inhibition. Nature 599, 679–683 (2021). Zhao et al. demonstrate that RAF1 is an essential gene for the proliferation of KRAS mutant cells that had acquired resistance to the KRAS G12C inhibitor, sotorasib.
Palma, G., Khurshid, F., Lu, K., Woodward, B. & Husain, H. Selective KRAS G12C inhibitors in non-small cell lung cancer: chemistry, concurrent pathway alterations, and clinical outcomes. NPJ Precis. Oncol. 5, 98 (2021).
Corral de la Fuente, E., Olmedo Garcia, M. E., Gomez Rueda, A., Lage, Y. & Garrido, P. Targeting KRAS in non-small cell lung cancer. Front. Oncol. 11, 792635 (2021).
Kim, D. et al. Pan-KRAS inhibitor disables oncogenic signalling and tumour growth. Nature 619, 160–166 (2023). This study describes a novel pan-KRAS mutant inhibitor that is effective at inhibiting the growth of a tumours with a wide array of KRAS mutations, including LS513 colorectal cancer cells with co-occurring KRAS G12D and RAF1 E478K mutations.
Touat, M. et al. Successful targeting of an ATG7-RAF1 gene fusion in anaplastic pleomorphic xanthoastrocytoma with leptomeningeal dissemination. JCO Precis. Oncol. 3, 1–7 (2019).
Kim, K. B. et al. Significant clinical response to a MEK inhibitor therapy in a patient with metastatic melanoma harboring an RAF1 fusion. JCO Precis. Oncol. 2, 1–6 (2018).
McEvoy, C. R. et al. MEK inhibitor therapy in carcinomas with RAF1 fusions: inferior response in a patient with pancreatic acinar cell carcinoma. JCO Precis. Oncol. 3, 1–2 (2019).
Goto, H. et al. Pancreatoblastoma with a novel fusion gene of IQSEC1-RAF1. Pediatr. Blood Cancer 70, e30155 (2023).
Boileau, M. et al. Clinical response under MEK inhibitor alone in metastatic melanoma with a novel fusion involving the RAF1 gene. Melanoma Res. 33, 247–251 (2023).
Panet, F., Jung, S. & Alcindor, T. Sustained response to the mitogen-activated extracellular kinase inhibitor trametinib in a spindle cell sarcoma harboring a QKI-RAF1 gene fusion. JCO Precis. Oncol. 6, e2100303 (2022).
Nakama, K. et al. Clinical response to a MEK inhibitor in a patient with metastatic melanoma harboring an RAF1 gene rearrangement detected by cancer gene panel testing. J. Dermatol. 48, e256–e257 (2021).
Nikanjam, M., Tinajero, J., Barkauskas, D. A. & Kurzrock, R. BRAF V600E/V600K mutations versus nonstandard alterations: prognostic implications and therapeutic outcomes. Mol. Cancer Ther. 20, 1072–1079 (2021).
Henry, J. R. et al. Discovery of 1-(3,3-dimethylbutyl)-3-(2-fluoro-4-methyl-5-(7-methyl-2-(methylamino)pyrido[2,3-d]pyrimidin-6-yl)phenyl)urea (LY3009120) as a pan-RAF inhibitor with minimal paradoxical activation and activity against BRAF or RAS mutant tumor cells. J. Med. Chem. 58, 4165–4179 (2015).
Wilhelm, S. M. et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 64, 7099–7109 (2004).
Wilhelm, S. M. et al. Regorafenib (BAY 73-4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int. J. Cancer 129, 245–255 (2011).
Yen, I. et al. ARAF mutations confer resistance to the RAF inhibitor belvarafenib in melanoma. Nature 594, 418–423 (2021). This study demonstrates that ARAF mutations can drive resistance to pan-RAF inhibitors and that pan-RAF and MEK inhibition is more effective than either monotherapy in NRAS mutant melanoma.
ClinicalTrials.gov. A Study to evaluate KIN-2787 in participants with BRAF and/or NRAS mutation positive solid tumors. US National Library of Medicine. http://www.clinicaltrials.gov/ct2/show/NCT04913285 (2023).
ClinicalTrials.gov. A study of JZP815 oral capsules in adult participants with advanced or metastatic solid tumors harboring mitogen activated protein kinase (MAPK) pathway alterations to investigate the safety, dosing, and antitumor activity of JZP815. US National Library of Medicine. http://www.clinicaltrials.gov/ct2/show/ NCT05557045 (2023).
ClinicalTrials.gov. Safety, tolerability and pharmacokinetics study of QLH11906 in patients with advanced solid tumors harboring MAPK pathway alterations. US National Library of Medicine. http://www.clinicaltrials.gov/ct2/show/NCT05488821 (2022).
de Braud, F. et al. Initial evidence for the efficacy of naporafenib in combination with trametinib in NRAS-mutant melanoma: results from the expansion arm of a phase ib, open-label study. J. Clin. Oncol. 41, 2651–2660 (2023). This is the first clinical trial, to our knowledge, reporting preliminary efficacy results of combination therapy with pan-RAF and MEK inhibitors in patients with RAS mutant cancer.
Fedele, C. et al. SHP2 inhibition prevents adaptive resistance to MEK inhibitors in multiple cancer models. Cancer Discov. 8, 1237–1249 (2018).
ClinicalTrials.gov. Tumor-Agnostic Precision Immuno-Oncology and Somatic Targeting Rational for You (TAPISTRY) platform study. US National Library of Medicine. http://www.clinicaltrials.gov/ct2/show/NCT04589845 (2023).
Sullivan, R. J. et al. A phase I study of LY3009120, a Pan-RAF inhibitor, in patients with advanced or metastatic cancer. Mol. Cancer Ther. 19, 460–467 (2020). This is the first published report, to our knowledge, of a clinical trial investigating a novel pan-RAF inhibitor.
Vakana, E. et al. LY3009120, a panRAF inhibitor, has significant anti-tumor activity in BRAF and KRAS mutant preclinical models of colorectal cancer. Oncotarget 8, 9251–9266 (2017).
Frederick, D. T. et al. BRAF inhibition is associated with enhanced melanoma antigen expression and a more favorable tumor microenvironment in patients with metastatic melanoma. Clin. Cancer Res. 19, 1225–1231 (2013).
Homet Moreno, B., Mok, S., Comin-Anduix, B., Hu-Lieskovan, S. & Ribas, A. Combined treatment with dabrafenib and trametinib with immune-stimulating antibodies for BRAF mutant melanoma. Oncoimmunology 5, e1052212 (2016).
Ascierto, P. A. et al. Dabrafenib, trametinib and pembrolizumab or placebo in BRAF-mutant melanoma. Nat. Med. 25, 941–946 (2019).
Sullivan, R. J. et al. Atezolizumab plus cobimetinib and vemurafenib in BRAF-mutated melanoma patients. Nat. Med. 25, 929–935 (2019).
Schramm, K., Niehof, M., Radziwill, G., Rommel, C. & Moelling, K. Phosphorylation of c-Raf-1 by protein kinase A interferes with activation. Biochem. Biophys. Res. Commun. 201, 740–747 (1994).
Kunapuli, P., Kasyapa, C. S., Hawthorn, L. & Cowell, J. K. LGI1, a putative tumor metastasis suppressor gene, controls in vitro invasiveness and expression of matrix metalloproteinases in glioma cells through the ERK1/2 pathway. J. Biol. Chem. 279, 23151–23157 (2004).
Zimmermann, S. & Moelling, K. Phosphorylation and regulation of Raf by Akt (protein kinase B). Science 286, 1741–1744 (1999).
Abraham, D. et al. Raf-1-associated protein phosphatase 2A as a positive regulator of kinase activation. J. Biol. Chem. 275, 22300–22304 (2000).
Morrison, D. K., Heidecker, G., Rapp, U. R. & Copeland, T. D. Identification of the major phosphorylation sites of the Raf-1 kinase. J. Biol. Chem. 268, 17309–17316 (1993).
Balan, V. et al. Identification of novel in vivo Raf-1 phosphorylation sites mediating positive feedback Raf-1 regulation by extracellular signal-regulated kinase. Mol. Biol. Cell 17, 1141–1153 (2006).
Zang, M., Hayne, C. & Luo, Z. Interaction between active Pak1 and Raf-1 is necessary for phosphorylation and activation of Raf-1. J. Biol. Chem. 277, 4395–4405 (2002).
Wu, X., Carr, H. S., Dan, I., Ruvolo, P. P. & Frost, J. A. p21 activated kinase 5 activates Raf-1 and targets it to mitochondria. J. Cell Biochem. 105, 167–175 (2008).
von Kriegsheim, A., Pitt, A., Grindlay, G. J., Kolch, W. & Dhillon, A. S. Regulation of the Raf-MEK-ERK pathway by protein phosphatase 5. Nat. Cell Biol. 8, 1011–1016 (2006).
Diaz, B. et al. Phosphorylation of Raf-1 serine 338-Serine 339 is an essential regulatory event for Ras-dependent activation and biological signaling. Mol. Cell. Biol. 17, 4509–4516 (1997).
Guo, J. et al. Sustained activation of Src-family tyrosine kinases by ischemia: a potential mechanism mediating extracellular signal-regulated kinase cascades in hippocampal dentate gyrus. Neuroscience 143, 827–836 (2006).
Carroll, M. P. & May, W. S. Protein kinase C-mediated serine phosphorylation directly activates Raf-1 in murine hematopoietic cells. J. Biol. Chem. 269, 1249–1256 (1994).
Kolch, W. et al. Protein kinase C alpha activates RAF-1 by direct phosphorylation. Nature 364, 249–252 (1993).
Hekman, M. et al. Dynamic changes in C-Raf phosphorylation and 14-3-3 protein binding in response to growth factor stimulation: differential roles of 14-3-3 protein binding sites. J. Biol. Chem. 279, 14074–14086 (2004).
Baek, K. H., Fabian, J. R., Sprenger, F., Morrison, D. K. & Ambrosio, L. The activity of D-raf in torso signal transduction is altered by serine substitution, N-terminal deletion, and membrane targeting. Dev. Biol. 175, 191–204 (1996).
Harms, F. L. et al. The novel RAF1 mutation p.(Gly361Ala) located outside the kinase domain of the CR3 region in two patients with Noonan syndrome, including one with a rare brain tumor. Am. J. Med. Genet. A 176, 470–476 (2018).
Boned Del Río, I. et al. SHOC2 complex-driven RAF dimerization selectively contributes to ERK pathway dynamics. Proc. Natl Acad. Sci. USA 116, 13330–13339 (2019).
Blum, A., Wang, P. & Zenklusen, J. C. SnapShot: TCGA-analyzed tumors. Cell 173, 530 (2018).
de Bruijn, I. et al. Analysis and visualization of longitudinal genomic and clinical data from the AACR project GENIE Biopharma Collaborative in cBioPortal. Cancer Res. 83, 3861–3867 (2023).
Cerami, E. et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2, 401–404 (2012).
ClinicalTrials.gov. Study of the safety and pharmacokinetics of BGB-283 (lifirafenib) and PD-0325901 (mirdametinib) in participants with advanced or refractory solid tumors. US National Library of Medicine. http://www.clinicaltrials.gov/ct2/show/NCT03905148 (2023).
Desai, J. et al. Phase I, open-label, dose-escalation/dose-expansion study of lifirafenib (BGB-283), an RAF family kinase inhibitor, in patients with solid tumors. J. Clin. Oncol. 38, 2140–2150 (2020).
ClinicalTrials.gov. A study to evaluate the safety and activity of belvarafenib as a single agent and in combination with either cobimetinib or cobimetinib plus nivolumab in patients with NRAS-mutant advanced melanoma. US National Library of Medicine. http://www.clinicaltrials.gov/ct2/show/NCT04835805 (2023).
ClinicalTrials.gov. HM95573 in combination with either cobimetinib or cetuximab in patients with locally advanced or metastatic solid tumors. US National Library of Medicine. http://www.clinicaltrials.gov/ct2/show/NCT03284502 (2023).
ClinicalTrials.gov. DAY101 in gliomas and other tumors. US National Library of Medicine. http://www.clinicaltrials.gov/ct2/show/NCT03429803 (2023).
ClinicalTrials.gov. A study to evaluate DAY101 in pediatric and young adult patients with relapsed or progressive low-grade glioma and advance solid tumors (FIREFLY-1). US National Library of Medicine. http://www.clinicaltrials.gov/ct2/show/NCT04775485 (2023).
ClinicalTrials.gov. Nivolumab and DAY101 for treatment of craniopharyngioma in children and toung adults (PNOC029). US National Library of Medicine. http://www.clinicaltrials.gov/ct2/show/NCT05465174 (2023).
ClinicalTrials.gov. Expanded Access Program (EAP) for tovorafenib (DAY101) in RAF-altered, relapsed or refractory low-grade glioma. US National Library of Medicine. http://www.clinicaltrials.gov/ct2/show/NCT05760586 (2023).
Rasco, D. W. et al. Phase 1 study of the pan-RAF inhibitor tovorafenib in patients with advanced solid tumors followed by dose expansion in patients with metastatic melanoma. Cancer Chemother. Pharmacol. 92, 15–28 (2023).
ClinicalTrials.gov. US National Library of Medicine. Expanded Access Program (EAP) for tovorafenib (DAY101) in RAF-altered, relapsed or refractory low-grade glioma. https://www.clinicaltrials.gov/study/NCT05760586 (2023).
ClinicalTrials.gov. A phase Ib study of LXH254-centric combinations in NSCLC or melanoma. US National Library of Medicine. http://www.clinicaltrials.gov/ct2/show/NCT02974725 (2023).
ClinicalTrials.gov. A study to assess naporafenib (ERAS-254) administered with trametinib in patients with RAS Q61X mutations (SEACRAFT-1). US National Library of Medicine. http://www.clinicaltrials.gov/ct2/show/NCT05907304 (2023).
ClinicalTrials.gov. Study of efficacy and safety of LXH254 combinations in patients with previously treated unresectable or metastatic melanoma. US National Library of Medicine. http://www.clinicaltrials.gov/ct2/show/NCT04417621 (2023).
McDonald, C. B., Seldeen, K. L., Deegan, B. J., Bhat, V. & Farooq, A. Assembly of the Sos1–Grb2–Gab1 ternary signaling complex is under allosteric control. Arch. Biochem. Biophys. 494, 216–225 (2010).
Stowe, I. B. et al. A shared molecular mechanism underlies the human rasopathies Legius syndrome and Neurofibromatosis-1. Genes. Dev. 26, 1421–1426 (2012).
Acknowledgements
M.R. and I.S.-B. are recipients of Canadian Institutes of Health Research (CIHR) postdoctoral fellowships, J.M. is the recipient of an Elizabeth Steffen Memorial Fellowship and M.D. is the recipient of a Vanier Canada Graduate Scholarship. A.A.N.R. acknowledges support from a Fonds de Recherche du Québec – Santé (FRQS) Clinical Research Scholar Award. Funding for this research was provided by a Canadian Cancer Society Challenge Grant (#707457), a TransMedTech Institute and Apogee Canada Research Excellence Fund and the Louise Couture (Chartier) Memorial Fund to A.A.N.R.
Author information
Authors and Affiliations
Contributions
M.R., J.M., I.S.-B., M.D. and A.A.N.R. wrote the article. M.R., J.M. and I.S.-B. contributed equally to all aspects of the article. All authors researched data for the article. M.R., J.M., I.S.-B., M.D. and A.A.N.R. contributed substantially to discussion of the content. All authors reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
I.S.-B. is an employee of Repare Therapeutics. A.A.N.R. reports research grants from the Canadian Institutes of Health Research (CIHR), the Canadian Cancer Society (CCS), Canada Foundation for Innovation (CFI) the Conquer Cancer Foundation of ASCO and Fonds de Recherche du Québec — Santé during the conduct of the study; personal fees from Advanced Accelerator Applications/Novartis and EMD Serono during the conduct of this study. A.A.N.R. reports institutional research funding for clinical research from: Bayer, Merck, Pfizer, AstraZeneca, and Seagen — not related to the submitted work. M.R., J.M., M.D. and M.L. have no competing interests.
Peer review
Peer review information
Nature Reviews Cancer thanks Grant McArthur and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
cBioportal: https://www.cbioportal.org/
Supplementary information
Glossary
- American Association for Cancer Research Genomics Evidence Neoplasia Information Exchange version 14
-
(AACR GENIE v14). A multiphase, multiyear, international data-sharing project that aims to catalyse precision cancer medicine.
- BRAF V600
-
The V600 mutation describes an amino acid substitution of the valine at position 600 in BRAF; the two most common mutations substitute a glutamic acid (E) or a lysine (K) amino acid resulting in a constitutively activated kinase.
- Cardiofaciocutaneous disease
-
A syndrome in which patients have multiple congenital anomalies or intellectual disabilities; failure to thrive; psychomotor delay; a characteristic face; congenital heart defects; and abnormalities of the skin, eyes, gastrointestinal tract and central nervous system.
- Histiocytic neoplasms
-
Hematologic disorders characterized by the accumulation of myeloid–dendritic cell-derived neoplastic cells with an accompanying inflammatory infiltrate; they account for less than 1% of cancers of the soft tissue and lymph nodes.
- Oncogenic hotspot mutations
-
Recurrently mutated DNA positions in cancer, which confer tumourigenic properties to the affected gene.
- Pheochromocytoma
-
A type of neuroendocrine tumour that originates from hormone-producing chromaffin cells within the adrenal glands.
- Phosphomimetic substitution
-
Amino acid substitutions, commonly at serine, tyrosine and threonine, by introducing an amino acid with a phosphate group that mimics the effect of a phosphorylated protein, thereby activating (or deactivating) the protein.
- Pilocytic astrocytomas
-
A family of slow-growing tumours that arise from glial cells that are mostly benign and thus are the most treatable type of the glioma with a cure rate of over 90%.
- Pleomorphic xanthoastrocytoma
-
A very rare type of astrocytoma, which arises in the brain or spinal cord.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Riaud, M., Maxwell, J., Soria-Bretones, I. et al. The role of CRAF in cancer progression: from molecular mechanisms to precision therapies. Nat Rev Cancer 24, 105–122 (2024). https://doi.org/10.1038/s41568-023-00650-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41568-023-00650-x
This article is cited by
-
ERK pathway agonism for cancer therapy: evidence, insights, and a target discovery framework
npj Precision Oncology (2024)