Abstract
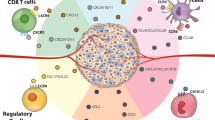
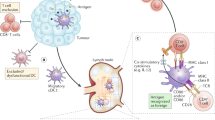
For our immune system to contain or eliminate malignant solid tumours, both myeloid and lymphoid haematopoietic cells must not only extravasate from the bloodstream into the tumour tissue but also further migrate to various specialized niches of the tumour microenvironment to functionally interact with each other, with non-haematopoietic stromal cells and, ultimately, with cancer cells. These interactions regulate local immune cell survival, proliferative expansion, differentiation and their execution of pro-tumour or antitumour effector functions, which collectively determine the outcome of spontaneous or therapeutically induced antitumour immune responses. None of these interactions occur randomly but are orchestrated and critically depend on migratory guidance cues provided by chemokines, a large family of chemotactic cytokines, and their receptors. Understanding the functional organization of the tumour immune microenvironment inevitably requires knowledge of the multifaceted roles of chemokines in the recruitment and positioning of its cellular constituents. Gaining such knowledge will not only generate new insights into the mechanisms underlying antitumour immunity or immune tolerance but also inform the development of biomarkers (or ‘biopatterns’) based on spatial tumour tissue analyses, as well as novel strategies to therapeutically engineer immune responses in patients with cancer. Here we will discuss recent observations on the role of chemokines in the tumour microenvironment in the context of our knowledge of their physiological functions in development, homeostasis and antimicrobial responses.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Galon, J. & Bruni, D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat. Rev. Drug Discov. 18, 197–218 (2019).
Puram, S. V. et al. Single-cell transcriptomic analysis of primary and metastatic tumor ecosystems in head and neck cancer. Cell 171, 1611–1624.e24 (2017).
Zhang, Q. et al. Landscape and dynamics of single immune cells in hepatocellular carcinoma. Cell 179, 829–845.e20 (2019).
Bill, R. et al. CXCL9:SPP1 macrophage polarity identifies a network of cellular programs that control human cancers. Science 381, 515–524 (2023).
Wang, R. et al. Single-cell dissection of intratumoral heterogeneity and lineage diversity in metastatic gastric adenocarcinoma. Nat. Med. 27, 141–151 (2021).
Zilionis, R. et al. Single-cell transcriptomics of human and mouse lung cancers reveals conserved myeloid populations across individuals and species. Immunity 50, 1317–1334.e10 (2019).
Binnewies, M. et al. Unleashing type-2 dendritic cells to drive protective antitumor CD4+ T cell immunity. Cell 177, 556–571.e16 (2019).
Scott, A. C. et al. TOX is a critical regulator of tumour-specific T cell differentiation. Nature 571, 270–274 (2019).
Davidson, S. et al. Single-cell RNA sequencing reveals a dynamic stromal niche that supports tumor growth. Cell Rep. 31, 107628 (2020).
Molgora, M. et al. TREM2 modulation remodels the tumor myeloid landscape enhancing anti-PD-1 immunotherapy. Cell 182, 886–900.e17 (2020).
Maier, B. et al. A conserved dendritic-cell regulatory program limits antitumour immunity. Nature 580, 257–262 (2020).
Di Pilato, M. et al. CXCR6 positions cytotoxic T cells to receive critical survival signals in the tumor microenvironment. Cell 184, 4512–4530.e22 (2021). This paper highlights how chemokines can promote immune cell survival and proliferation in the TME indirectly by guiding them to engage in cellular interactions.
Morein, D., Erlichman, N. & Ben-Baruch, A. Beyond cell motility: the expanding roles of chemokines and their receptors in malignancy. Front. Immunol. 11, 952 (2020).
Rot, A. & von Andrian, U. H. Chemokines in innate and adaptive host defense: basic chemokinese grammar for immune cells. Annu. Rev. Immunol. 22, 891–928 (2004). This study is an insightful comparison of the chemokine system to a language.
Chow, M. T. et al. Intratumoral activity of the CXCR3 chemokine system is required for the efficacy of anti-PD-1 therapy. Immunity 50, 1498–1512.e5 (2019).
Mikucki, M. E. et al. Non-redundant requirement for CXCR3 signalling during tumoricidal T-cell trafficking across tumour vascular checkpoints. Nat. Commun. 6, 7458 (2015). This study is the first direct demonstration of a role for a chemokine in immune cell extravasation to tumours.
Marangoni, F. et al. Tumor tolerance-promoting function of regulatory T cells is optimized by CD28, but strictly dependent on calcineurin. J. Immunol. 200, 3647–3661 (2018).
Moreno Ayala, M. A. et al. CXCR3 expression in regulatory T cells drives interactions with type I dendritic cells in tumors to restrict CD8+ T cell antitumor immunity. Immunity 56, 1613–1630.e5 (2023).
González-Martín, A., Gómez, L., Lustgarten, J., Mira, E. & Mañes, S. Maximal T cell-mediated antitumor responses rely upon CCR5 expression in both CD4+ and CD8+ T cells. Cancer Res. 71, 5455–5466 (2011).
Walens, A. et al. CCL5 promotes breast cancer recurrence through macrophage recruitment in residual tumors. eLife 8, e43653 (2019).
Rozhok, A. I. & DeGregori, J. The evolution of lifespan and age-dependent cancer risk. Trends Cancer 2, 552–560 (2016).
Foster, A. D., Sivarapatna, A. & Gress, R. E. The aging immune system and its relationship with cancer. Aging Health 7, 707–718 (2011).
Zlotnik, A. & Yoshie, O. Chemokines: a new classification system and their role in immunity. Immunity 12, 121–127 (2000).
Bachelerie, F. et al. International union of basic and clinical pharmacology. LXXXIX. Update on the extended family of chemokine receptors and introducing a new nomenclature for atypical chemokine receptors. Pharmacol. Rev. 66, 1–79 (2014). This study is a comprehensive and systematic overview of the chemokine system.
Zlotnik, A., Yoshie, O. & Nomiyama, H. The chemokine and chemokine receptor superfamilies and their molecular evolution. Genome Biol. 7, 243 (2006).
Bonecchi, R. & Graham, G. J. Atypical chemokine receptors and their roles in the resolution of the inflammatory response. Front. Immunol. 7, 224 (2016).
Murphy, P. M. The molecular biology of leukocyte chemoattractant receptors. Annu. Rev. Immunol. 12, 593–633 (1994).
Lämmermann, T. & Kastenmüller, W. Concepts of GPCR‐controlled navigation in the immune system. Immunol. Rev. 289, 205–231 (2019). This study is a review of the in vivo mechanisms of chemotactic cell guidance.
Teicher, B. A. & Fricker, S. P. CXCL12 (SDF-1)/CXCR4 pathway in cancer. Clin. Cancer Res. 16, 2927–2931 (2010).
Smith, J. S., Lefkowitz, R. J. & Rajagopal, S. Biased signalling: from simple switches to allosteric microprocessors. Nat. Rev. Drug Discov. 17, 243–260 (2018).
Crijns, H., Vanheule, V. & Proost, P. Targeting chemokine — glycosaminoglycan interactions to inhibit inflammation. Front. Immunol. 11, 483 (2020).
Ley, K., Laudanna, C., Cybulsky, M. I. & Nourshargh, S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat. Rev. Immunol. 7, 678–689 (2007).
Wang, J. & Knaut, H. Chemokine signaling in development and disease. Development 141, 4199–4205 (2014).
Schall, T. J. & Proudfoot, A. E. I. Overcoming hurdles in developing successful drugs targeting chemokine receptors. Nat. Rev. Immunol. 11, 355–363 (2011).
Murphy, P. M. Viral exploitation and subversion of the immune system through chemokine mimicry. Nat. Immunol. 2, 116–122 (2001).
D’Ambrosio, D. et al. Quantitative differences in chemokine receptor engagement generate diversity in integrin-dependent lymphocyte adhesion. J. Immunol. 169, 2303–2312 (2002).
Mariani, M., Lang, R., Binda, E., Panina-Bordignon, P. & D’Ambrosio, D. Dominance of CCL22 over CCL17 in induction of chemokine receptor CCR4 desensitization and internalization on human Th2 cells. Eur. J. Immunol. 34, 231–240 (2004).
Harlin, H. et al. Chemokine expression in melanoma metastases associated with CD8+ T-cell recruitment. Cancer Res. 69, 3077–3085 (2009).
Chheda, Z. S., Sharma, R. K., Jala, V. R., Luster, A. D. & Haribabu, B. Chemoattractant receptors BLT1 and CXCR3 regulate antitumor immunity by facilitating CD8+ T cell migration into tumors. J. Immunol. 197, 2016–2026 (2016).
Wendel, M., Galani, I. E., Suri-Payer, E. & Cerwenka, A. Natural killer cell accumulation in tumors is dependent on IFN-γ and CXCR3 ligands. Cancer Res. 68, 8437–8445 (2008).
Luster, A. D., Unkeless, J. C. & Ravetch, J. V. Gamma-interferon transcriptionally regulates an early-response gene containing homology to platelet proteins. Nature 315, 672–676 (1985).
Groom, J. R. & Luster, A. D. CXCR3 ligands: redundant, collaborative and antagonistic functions. Immunol. Cell Biol. 89, 207–215 (2011).
Bronger, H. et al. CXCL9 and CXCL10 predict survival and are regulated by cyclooxygenase inhibition in advanced serous ovarian cancer. Br. J. Cancer 115, 553–563 (2016).
Duckworth, B. C. et al. Effector and stem-like memory cell fates are imprinted in distinct lymph node niches directed by CXCR3 ligands. Nat. Immunol. 22, 434–448 (2021).
Groom, J. R. et al. CXCR3 chemokine receptor–ligand interactions in the lymph node optimize CD4+ T helper 1 cell differentiation. Immunity 37, 1091–1103 (2012).
Kastenmüller, W. et al. Peripheral prepositioning and local CXCL9 chemokine-mediated guidance orchestrate rapid memory CD8 + T cell responses in the lymph node. Immunity 38, 502–513 (2013).
Sung, J. H. et al. Chemokine guidance of central memory T cells is critical for antiviral recall responses in lymph nodes. Cell 150, 1249–1263 (2012).
Hickman, H. D. et al. CXCR3 chemokine receptor enables local CD8+ T cell migration for the destruction of virus-infected cells. Immunity 42, 524–537 (2015).
Ariotti, S. et al. Subtle CXCR3-dependent chemotaxis of CTLs within infected tissue allows efficient target localization. J. Immunol. 195, 5285–5295 (2015).
Bangs, D. J. et al. CXCR3 regulates stem and proliferative CD8+ T cells during chronic infection by promoting interactions with DCs in splenic bridging channels. Cell Rep. 38, 110266 (2022).
Dähling, S. et al. Type 1 conventional dendritic cells maintain and guide the differentiation of precursors of exhausted T cells in distinct cellular niches. Immunity 55, 656–670.e8 (2022).
Ozga, A. J. et al. CXCL10 chemokine regulates heterogeneity of the CD8+ T cell response and viral set point during chronic infection. Immunity 55, 82–97.e8 (2022).
Miller, B. C. et al. Subsets of exhausted CD8+ T cells differentially mediate tumor control and respond to checkpoint blockade. Nat. Immunol. 20, 326–336 (2019).
Siddiqui, I. et al. Intratumoral Tcf1 + PD-1 + CD8+ T cells with stem-like properties promote tumor control in response to vaccination and checkpoint blockade immunotherapy. Immunity 50, 195–211.e10 (2019).
Meiser, P. et al. A distinct stimulatory cDC1 subpopulation amplifies CD8+ T cell responses in tumors for protective anti-cancer immunity. Cancer Cell 41, 1498–1515.e10 (2023).
Dorner, B. G. et al. Selective expression of the chemokine receptor XCR1 on cross-presenting dendritic cells determines cooperation with CD8+ T cells. Immunity 31, 823–833 (2009). This paper demonstrates a previously elusive role of XCR1 in CD8+ T cell activation.
Hildner, K. et al. Batf3 deficiency reveals a critical role for CD8alpha + dendritic cells in cytotoxic T cell immunity. Science 322, 1097–1100 (2008).
Eickhoff, S. et al. Robust anti-viral immunity requires multiple distinct T cell-dendritic cell interactions. Cell 162, 1322–1337 (2015).
Hor, J. L. et al. Spatiotemporally distinct interactions with dendritic cell subsets facilitates CD4+ and CD8+ T cell activation to localized viral infection. Immunity 43, 554–565 (2015).
Kitano, M. et al. Imaging of the cross-presenting dendritic cell subsets in the skin-draining lymph node. Proc. Natl Acad. Sci. USA 113, 1044–1049 (2016). This study, along with Eickhoff et al. (2015) and Hor et al. (2015), uses state-of-the-art intravital microscopy techniques to resolve complex patterns of cooperativity in DC–T cell interactions in lymph nodes.
Bottcher, J. P. et al. NK cells stimulate recruitment of cDC1 into the tumor microenvironment promoting cancer immune control. Cell 172, 1022–1037.e14 (2018). This paper presents a key advance in understanding cancer cell-initiated immune-suppression mediated by interference with chemokine function.
Traynor, T. R., Kuziel, W. A., Toews, G. B. & Huffnagle, G. B. CCR2 expression determines T1 versus T2 polarization during pulmonary Cryptococcus neoformans infection. J. Immunol. 164, 2021–2027 (2000).
Blease, K. et al. Enhanced pulmonary allergic responses to Aspergillus in CCR2−/− mice. J. Immunol. 165, 2603–2611 (2000).
Luther, S. A. & Cyster, J. G. Chemokines as regulators of T cell differentiation. Nat. Immunol. 2, 102–107 (2001).
Castellino, F. et al. Chemokines enhance immunity by guiding naive CD8+ T cells to sites of CD4+ T cell–dendritic cell interaction. Nature 440, 890–895 (2006). This study demonstrates an elegant solution to the ‘three cell problem’ in the provision of CD4+ T cell help to CD8+ T cells.
Hickman, H. D. et al. Chemokines control naive CD8+ T cell selection of optimal lymph node antigen presenting cells. J. Exp. Med. 208, 2511–2524 (2011).
Galeano Niño, J. L. et al. Cytotoxic T cells swarm by homotypic chemokine signalling. eLife 9, e56554 (2020).
Weigelin, B. et al. Cytotoxic T cells are able to efficiently eliminate cancer cells by additive cytotoxicity. Nat. Commun. 12, 5217 (2021).
Gause, W. C., Wynn, T. A. & Allen, J. E. Type 2 immunity and wound healing: evolutionary refinement of adaptive immunity by helminths. Nat. Rev. Immunol. 13, 607–614 (2013).
Mattes, J. et al. Immunotherapy of cytotoxic T cell-resistant tumors by T helper 2 cells. J. Exp. Med. 197, 387–393 (2003).
Carretero, R. et al. Eosinophils orchestrate cancer rejection by normalizing tumor vessels and enhancing infiltration of CD8(+) T cells. Nat. Immunol. 16, 609–617 (2015).
Liu, M. et al. TGF-β suppresses type 2 immunity to cancer. Nature 587, 115–120 (2020).
Hollande, C. et al. Inhibition of the dipeptidyl peptidase DPP4 (CD26) reveals IL-33-dependent eosinophil-mediated control of tumor growth. Nat. Immunol. 20, 257–264 (2019).
Loetscher, P. et al. The ligands of CXC chemokine receptor 3, I-TAC, Mig, and IP10, are natural antagonists for CCR3. J. Biol. Chem. 276, 2986–2991 (2001).
Reboldi, A. et al. IgA production requires B cell interaction with subepithelial dendritic cells in Peyer’s patches. Science 352, aaf4822 (2016).
Le Borgne, M. et al. Dendritic cells rapidly recruited into epithelial tissues via CCR6/CCL20 are responsible for CD8+ T cell crosspriming in vivo. Immunity 24, 191–201 (2006).
Bruchard, M. et al. Recruitment and activation of type 3 innate lymphoid cells promote antitumor immune responses. Nat. Immunol. 23, 262–274 (2022).
Szabo, P. A., Miron, M. & Farber, D. L. Location, location, location: tissue resident memory T cells in mice and humans. Sci. Immunol. 4, eaas9673 (2019).
Geissmann, F. et al. Intravascular immune surveillance by CXCR6+ NKT cells patrolling liver sinusoids. PLoS Biol. 3, e113 (2005).
Paust, S. et al. Critical role for the chemokine receptor CXCR6 in NK cell-mediated antigen-specific memory of haptens and viruses. Nat. Immunol. 11, 1127–1135 (2010).
Shimaoka, T. et al. Cell surface-anchored SR-PSOX/CXC chemokine ligand 16 mediates firm adhesion of CXC chemokine receptor 6-expressing cells. J. Leukoc. Biol. 75, 267–274 (2004).
Abel, S. et al. The transmembrane CXC-chemokine ligand 16 is induced by IFN-gamma and TNF-alpha and shed by the activity of the disintegrin-like metalloproteinase ADAM10. J. Immunol. 172, 6362–6372 (2004).
Darash-Yahana, M. et al. The chemokine CXCL16 and its receptor, CXCR6, as markers and promoters of inflammation-associated cancers. PLoS ONE 4, e6695 (2009).
Gutwein, P. et al. Tumoural CXCL16 expression is a novel prognostic marker of longer survival times in renal cell cancer patients. Eur. J. Cancer 45, 478–489 (2009).
Hojo, S. et al. High-level expression of chemokine CXCL16 by tumor cells correlates with a good prognosis and increased tumor-infiltrating lymphocytes in colorectal cancer. Cancer Res. 67, 4725–4731 (2007).
Muthuswamy, R. et al. CXCR6 by increasing retention of memory CD8 + T cells in the ovarian tumor microenvironment promotes immunosurveillance and control of ovarian cancer. J. Immunother. Cancer 9, e003329 (2021).
Matsumura, S. et al. Radiation-induced CXCL16 release by breast cancer cells attracts effector T cells. J. Immunol. 181, 3099–3107 (2008).
Oldham, K. A. et al. T lymphocyte recruitment into renal cell carcinoma tissue: a role for chemokine receptors CXCR3, CXCR6, CCR5, and CCR6. Eur. Urol. 61, 385–394 (2012).
Parsonage, G. et al. CXCR6 and CCR5 localize T lymphocyte subsets in nasopharyngeal carcinoma. Am. J. Pathol. 180, 1215–1222 (2012).
Jerby-Arnon, L. et al. Pan-cancer mapping of single T cell profiles reveals a TCF1:CXCR6-CXCL16 regulatory axis essential for effective anti-tumor immunity. Preprint at bioRxiv https://doi.org/10.1101/2021.10.31.466532 (2021).
Karaki, S. et al. CXCR6 deficiency impairs cancer vaccine efficacy and CD8+ resident memory T-cell recruitment in head and neck and lung tumors. J. Immunother. Cancer 9, e001948 (2021).
Vella, J. L. et al. Dendritic cells maintain anti-tumor immunity by positioning CD8 skin-resident memory T cells. Life Sci. Alliance 4, e202101056 (2021).
Wang, B. et al. CXCR6 is required for antitumor efficacy of intratumoral CD8(+) T cell. J. Immunother. Cancer 9, e003100 (2021).
Rivas-Fuentes, S., Salgado-Aguayo, A., Arratia-Quijada, J. & Gorocica-Rosete, P. Regulation and biological functions of the CX3CL1–CX3CR1 axis and its relevance in solid cancer: a mini-review. J. Cancer 12, 571–583 (2021).
Hochreiter-Hufford, A. & Ravichandran, K. S. Clearing the dead: apoptotic cell sensing, recognition, engulfment, and digestion. Cold Spring Harb. Perspect. Biol. 5, a008748 (2013).
Sheridan, G. K. & Murphy, K. J. Neuron–glia crosstalk in health and disease: fractalkine and CX 3 CR1 take centre stage. Open Biol. 3, 130181 (2013).
Cormican, S. & Griffin, M. D. Fractalkine (CX3CL1) and its receptor CX3CR1: a promising therapeutic target in chronic kidney disease? Front. Immunol. 12, 664202 (2021).
Imai, T. et al. Identification and molecular characterization of fractalkine receptor CX3CR1, which mediates both leukocyte migration and adhesion. Cell 91, 521–530 (1997).
Jung, S. et al. Analysis of fractalkine receptor CX 3 CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol. Cell Biol. 20, 4106–4114 (2000).
Gerlach, C. et al. The chemokine receptor CX3CR1 defines three antigen-experienced CD8 T cell subsets with distinct roles in immune surveillance and homeostasis. Immunity 45, 1270–1284 (2016).
Böttcher, J. P. et al. Functional classification of memory CD8+ T cells by CX 3 CR1 expression. Nat. Commun. 6, 8306 (2015).
Hudson, W. H. et al. Proliferating transitory T cells with an effector-like transcriptional signature emerge from PD-1(+) stem-like CD8(+) T cells during chronic infection. Immunity 51, 1043–1058.e4 (2019).
Zander, R. et al. CD4(+) T cell help is required for the formation of a cytolytic CD8(+) T cell subset that protects against chronic infection and cancer. Immunity 51, 1028–1042.e4 (2019).
Yamauchi, T. et al. CX3CR1-CD8+ T cells are critical in antitumor efficacy but functionally suppressed in the tumor microenvironment. JCI Insight 5, e133920 (2020).
Yamauchi, T. et al. T-cell CX3CR1 expression as a dynamic blood-based biomarker of response to immune checkpoint inhibitors. Nat. Commun. 12, 1402 (2021).
Siddiqui, I., Erreni, M., van Brakel, M., Debets, R. & Allavena, P. Enhanced recruitment of genetically modified CX3CR1-positive human T cells into fractalkine/CX3CL1 expressing tumors: importance of the chemokine gradient. J. Immunother. Cancer 4, 21 (2016).
Kee, J.-Y. et al. Antitumor immune activity by chemokine CX3CL1 in an orthotopic implantation of lung cancer model in vivo. Mol. Clin. Oncol. 1, 35–40 (2013).
Batista, N. V. et al. T cell–intrinsic CX3CR1 marks the most differentiated effector CD4 + T cells, but is largely dispensable for CD4 + T cell responses during chronic viral infection. Immunohorizons 4, 701–712 (2020).
Gordon, C. L. et al. Induction and maintenance of CX3CR1-intermediate peripheral memory CD8(+) T cells by persistent viruses and vaccines. Cell Rep. 23, 768–782 (2018).
Sakai, S. et al. Cutting edge: control of Mycobacterium tuberculosis infection by a subset of lung parenchyma–homing CD4 T cells. J. Immunol. 192, 2965–2969 (2014).
Auffray, C. et al. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science 317, 666–670 (2007).
Konradt, C. & Hunter, C. A. Pathogen interactions with endothelial cells and the induction of innate and adaptive immunity. Eur. J. Immunol. 48, 1607–1620 (2018).
Campbell, D. J. & Koch, M. A. Phenotypical and functional specialization of FOXP3+ regulatory T cells. Nat. Rev. Immunol. 11, 119–130 (2011).
Mempel, T. R. & Marangoni, F. Guidance factors orchestrating regulatory T cell positioning in tissues during development, homeostasis, and response. Immunol. Rev. 289, 129–141 (2019).
Wang, L. et al. Connecting blood and intratumoral Treg cell activity in predicting future relapse in breast cancer. Nat. Immunol. 20, 1220–1230 (2019).
Curiel, T. J. et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat. Med. 10, 942–949 (2004).
Rapp, M. et al. CCL22 controls immunity by promoting regulatory T cell communication with dendritic cells in lymph nodes. J. Exp. Med. 216, 1170–1181 (2019).
Zhang, L. et al. Single-cell analyses inform mechanisms of myeloid-targeted therapies in colon cancer. Cell 181, 442–459.e29 (2020).
Marangoni, F. et al. Expansion of tumor-associated Treg cells upon disruption of a CTLA-4-dependent feedback loop. Cell 184, 3998–4015.e19 (2021).
Plitas, G. et al. Regulatory T cells exhibit distinct features in human breast cancer. Immunity 45, 1122–1134 (2016).
De Simone, M. et al. Transcriptional landscape of human tissue lymphocytes unveils uniqueness of tumor-infiltrating T regulatory cells. Immunity 45, 1135–1147 (2016).
Mikhak, Z. et al. Contribution of CCR4 and CCR8 to antigen-specific TH2 cell trafficking in allergic pulmonary inflammation. J. Allergy Clin. Immunol. 123, 67–73.e3 (2009).
Islam, S. A. et al. Mouse CCL8, a CCR8 agonist, promotes atopic dermatitis by recruiting IL-5 + TH2 cells. Nat. Immunol. 12, 167–177 (2011).
Sokol, C. L., Camire, R. B., Jones, M. C. & Luster, A. D. The chemokine receptor CCR8 promotes the migration of dendritic cells into the lymph node parenchyma to initiate the allergic immune response. Immunity 49, 449–463.e6 (2018).
Cucolo, L. et al. The interferon-stimulated gene RIPK1 regulates cancer cell intrinsic and extrinsic resistance to immune checkpoint blockade. Immunity 55, 671–685.e10 (2022).
Eberlein, J. et al. Chemokine signatures of pathogen-specific T cells I: effector T cells. J. Immunol. 205, 2169–2187 (2020).
Li, A. et al. IL-33 signaling alters regulatory T cell diversity in support of tumor development. Cell Rep. 29, 2998–3008.e8 (2019).
Eash, K. J., Greenbaum, A. M., Gopalan, P. K. & Link, D. C. CXCR2 and CXCR4 antagonistically regulate neutrophil trafficking from murine bone marrow. J. Clin. Invest. 120, 2423–2431 (2010).
Köhler, A. et al. G-CSF-mediated thrombopoietin release triggers neutrophil motility and mobilization from bone marrow via induction of Cxcr2 ligands. Blood 117, 4349–4357 (2011).
Girbl, T. et al. Distinct compartmentalization of the chemokines CXCL1 and CXCL2 and the atypical receptor ACKR1 determine discrete stages of neutrophil diapedesis. Immunity 49, 1062–1076.e6 (2018).
Chao, T., Furth, E. E. & Vonderheide, R. H. CXCR2-dependent accumulation of tumor-associated neutrophils regulates T-cell immunity in pancreatic ductal adenocarcinoma. Cancer Immunol. Res. 4, 968–982 (2016).
Seifert, L. et al. The necrosome promotes pancreatic oncogenesis via CXCL1 and Mincle-induced immune suppression. Nature 532, 245–249 (2016).
Li, J. et al. Tumor cell-intrinsic factors underlie heterogeneity of immune cell infiltration and response to immunotherapy. Immunity 49, 178–193.e7 (2018).
Acharyya, S. et al. A CXCL1 paracrine network links cancer chemoresistance and metastasis. Cell 150, 165–178 (2012).
Teijeira, Á. et al. CXCR1 and CXCR2 chemokine receptor agonists produced by tumors induce neutrophil extracellular traps that interfere with immune cytotoxicity. Immunity 52, 856–871.e8 (2020).
Kryczek, I. et al. Inflammatory regulatory T cells in the microenvironments of ulcerative colitis and colon carcinoma. OncoImmunol 5, e1105430 (2016).
Tecchio, C. & Cassatella, M. A. Neutrophil-derived chemokines on the road to immunity. Semin. Immunol. 28, 119–128 (2016).
Zhou, S.-L. et al. Tumor-associated neutrophils recruit macrophages and T-regulatory cells to promote progression of hepatocellular carcinoma and resistance to sorafenib. Gastroenterology 150, 1646–1658.e17 (2016).
Mishalian, I. et al. Neutrophils recruit regulatory T-cells into tumors via secretion of CCL17 — a new mechanism of impaired antitumor immunity. Int. J. Cancer 135, 1178–1186 (2014).
Granot, Z. et al. Tumor entrained neutrophils inhibit seeding in the premetastatic lung. Cancer Cell 20, 300–314 (2011).
Patel, A. A. et al. The fate and lifespan of human monocyte subsets in steady state and systemic inflammation. J. Exp. Med. 214, 1913–1923 (2017). This paper provides a clear demonstration that presumed cellular ‘subsets’ can represent stages of a linear differentiation process.
Yona, S. et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity 38, 79–91 (2013).
Pittet, M. J., Michielin, O. & Migliorini, D. Clinical relevance of tumour-associated macrophages. Nat. Rev. Clin. Oncol. 19, 402–421 (2022).
Roehle, K. et al. cIAP1/2 antagonism eliminates MHC class I-negative tumors through T cell-dependent reprogramming of mononuclear phagocytes. Sci. Transl. Med. 13, 23–25 (2021).
Li, X. et al. Targeting of tumour-infiltrating macrophages via CCL2/CCR2 signalling as a therapeutic strategy against hepatocellular carcinoma. Gut 66, 157–167 (2017).
Loberg, R. D. et al. Targeting CCL2 with systemic delivery of neutralizing antibodies induces prostate cancer tumor regression in vivo. Cancer Res. 67, 9417–9424 (2007).
Mitchem, J. B. et al. Targeting tumor-infiltrating macrophages decreases tumor-initiating cells, relieves immunosuppression, and improves chemotherapeutic responses. Cancer Res. 73, 1128–1141 (2013).
Qian, B.-Z. et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature 475, 222–225 (2011). This study presents the critical role of CCR2 in monocyte recruitment to tumours.
Sanford, D. E. et al. Inflammatory monocyte mobilization decreases patient survival in pancreatic cancer: a role for targeting the CCL2/CCR2 axis. Clin. Cancer Res. 19, 3404–3415 (2013).
Dyer, D. P. et al. Chemokine receptor redundancy and specificity are context dependent. Immunity 50, 378–389.e5 (2019).
Serbina, N. V. & Pamer, E. G. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat. Immunol. 7, 311–317 (2006).
Geissmann, F., Jung, S. & Littman, D. R. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 19, 71–82 (2003).
Trzebanski, S. & Jung, S. Plasticity of monocyte development and monocyte fates. Immunol. Lett. 227, 66–78 (2020).
Hanna, R. N. et al. Patrolling monocytes control tumor metastasis to the lung. Science 350, 985–990 (2015).
Jung, K. et al. Ly6Clo monocytes drive immunosuppression and confer resistance to anti-VEGFR2 cancer therapy. J. Clin. Invest. 127, 3039–3051 (2017).
Fantuzzi, L. et al. Loss of CCR2 expression and functional response to monocyte chemotactic protein (MCP-1) during the differentiation of human monocytes: role of secreted MCP-1 in the regulation of the chemotactic response. Blood 94, 875–883 (1999).
Kaufmann, A., Salentin, R., Gemsa, D. & Sprenger, H. Increase of CCR1 and CCR5 expression and enhanced functional response to MIP‐1α during differentiation of human monocytes to macrophages. J. Leukoc. Biol. 69, 248–252 (2001).
Medina-Ruiz, L. et al. Analysis of combinatorial chemokine receptor expression dynamics using multi-receptor reporter mice. eLife 11, e72418 (2022).
Halama, N. et al. Tumoral immune cell exploitation in colorectal cancer metastases can be targeted effectively by anti-CCR5 therapy in cancer patients. Cancer Cell 29, 587–601 (2016).
Frankenberger, C. et al. Metastasis suppressors regulate the tumor microenvironment by blocking recruitment of prometastatic tumor-associated macrophages. Cancer Res. 75, 4063–4073 (2015).
Braun, A. et al. Afferent lymph-derived T cells and DCs use different chemokine receptor CCR7–dependent routes for entry into the lymph node and intranodal migration. Nat. Immunol. 12, 879–887 (2011).
Roberts, E. W. et al. Critical role for CD103(+)/CD141(+) dendritic cells bearing CCR7 for tumor antigen trafficking and priming of T cell immunity in melanoma. Cancer Cell 30, 324–336 (2016).
Worbs, T., Mempel, T. R., Bölter, J., von Andrian, U. H. & Förster, R. CCR7 ligands stimulate the intranodal motility of T lymphocytes in vivo. J. Exp. Med. 204, 489–495 (2007).
Riedel, A., Shorthouse, D., Haas, L., Hall, B. A. & Shields, J. Tumor-induced stromal reprogramming drives lymph node transformation. Nat. Immunol. 17, 1118–1127 (2016).
Mueller, S. N. et al. Regulation of homeostatic chemokine expression and cell trafficking during immune responses. Science 317, 670–674 (2007).
Villablanca, E. J. et al. Tumor-mediated liver X receptor-α activation inhibits CC chemokine receptor-7 expression on dendritic cells and dampens antitumor responses. Nat. Med. 16, 98–105 (2010).
Petitprez, F. et al. B cells are associated with survival and immunotherapy response in sarcoma. Nature 577, 556–560 (2020).
Helmink, B. A. et al. B cells and tertiary lymphoid structures promote immunotherapy response. Nature 577, 549–555 (2020).
Cabrita, R. et al. Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature 577, 561–565 (2020).
Schumacher, T. N. & Thommen, D. S. Tertiary lymphoid structures in cancer. Science 375, eabf9419 (2022).
Shields, J. D., Kourtis, I. C., Tomei, A. A., Roberts, J. M. & Swartz, M. A. Induction of lymphoidlike stroma and immune escape by tumors that express the chemokine CCL21. Science 328, 749–752 (2010).
Joshi, N. S. et al. Regulatory T cells in tumor-associated tertiary lymphoid structures suppress anti-tumor T cell responses. Immunity 43, 579–590 (2015). This paper highlights that TLSs can also support tolerogenic function.
Pelka, K. et al. Spatially organized multicellular immune hubs in human colorectal cancer. Cell 184, 4734–4752.e20 (2021).
Thommen, D. S. et al. A transcriptionally and functionally distinct PD-1(+) CD8(+) T cell pool with predictive potential in non-small-cell lung cancer treated with PD-1 blockade. Nat. Med. 24, 994–1004 (2018).
Litchfield, K. et al. Meta-analysis of tumor- and T cell-intrinsic mechanisms of sensitization to checkpoint inhibition. Cell 184, 596–614.e14 (2021).
Wurbel, M.-A. et al. The chemokine TECK is expressed by thymic and intestinal epithelial cells and attracts double- and single-positive thymocytes expressing the TECK receptor CCR9. Eur. J. Immunol. 30, 262–271 (2000).
Chen, H. J. et al. Chemokine 25-induced signaling suppresses colon cancer invasion and metastasis. J. Clin. Invest. 122, 3184–3196 (2012).
Chen, H. et al. Intratumoral delivery of CCL25 enhances immunotherapy against triple-negative breast cancer by recruiting CCR9+ T cells. Sci. Adv. 6, eaax4690 (2020).
Bianchi, M. E. & Mezzapelle, R. The chemokine receptor CXCR4 in cell proliferation and tissue regeneration. Front. Immunol. 11, 2109 (2020).
Bonavita, O., Mollica Poeta, V., Massara, M., Mantovani, A. & Bonecchi, R. Regulation of hematopoiesis by the chemokine system. Cytokine 109, 76–80 (2018).
Sugiyama, T., Kohara, H., Noda, M. & Nagasawa, T. Maintenance of the hematopoietic stem cell pool by CXCL12–CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity 25, 977–988 (2006).
Okada, T. et al. Chemokine requirements for B cell entry to lymph nodes and Peyer’s patches. J. Exp. Med. 196, 65–75 (2002).
Scimone, M. L. et al. CXCL12 mediates CCR7-independent homing of central memory cells, but not naive T cells, in peripheral lymph nodes. J. Exp. Med. 199, 1113–1120 (2004).
Victora, G. D. et al. Germinal center dynamics revealed by multiphoton microscopy with a photoactivatable fluorescent reporter. Cell 143, 592–605 (2010). This study showcases a technical milestone that enabled the in vivo roles of chemokines in fine-tuned microenvironmental immune cell positioning to be pinpointed.
Ostrand-Rosenberg, S. & Fenselau, C. Myeloid-derived suppressor cells: immune-suppressive cells that impair antitumor immunity and are sculpted by their environment. J. Immunol. 200, 422–431 (2018).
Chiodoni, C. et al. Transcriptional profiles and stromal changes reveal bone marrow adaptation to early breast cancer in association with deregulated circulating microRNAs. Cancer Res. 80, 484–498 (2020).
Hoch, T. et al. Multiplexed imaging mass cytometry of the chemokine milieus in melanoma characterizes features of the response to immunotherapy. Sci. Immunol. 7, eabk1692 (2022).
Feig, C. et al. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc. Natl Acad. Sci. USA 110, 20212–20217 (2013).
Arwert, E. N. et al. A unidirectional transition from migratory to perivascular macrophage is required for tumor cell intravasation. Cell Rep. 23, 1239–1248 (2018).
Steele, M. M. et al. T cell egress via lymphatic vessels is tuned by antigen encounter and limits tumor control. Nat. Immunol. 24, 664–675 (2023).
Chen, Y. et al. CXCR4 inhibition in tumor microenvironment facilitates anti-programmed death receptor-1 immunotherapy in sorafenib-treated hepatocellular carcinoma in mice: hepatobiliary malignancies. Hepatology 61, 1591–1602 (2015).
Seo, Y. D. et al. Mobilization of CD8+ T cells via CXCR4 blockade facilitates PD-1 checkpoint therapy in human pancreatic cancer. Clin. Cancer Res. 25, 3934–3945 (2019).
Bockorny, B. et al. BL-8040, a CXCR4 antagonist, in combination with pembrolizumab and chemotherapy for pancreatic cancer: the COMBAT trial. Nat. Med. 26, 878–885 (2020). This paper demonstrates that chemokine targeting can have clinical anti-cancer efficacy.
Müller, A. et al. Involvement of chemokine receptors in breast cancer metastasis. Nature 410, 50–56 (2001). This study demonstrates the role of chemokines in guiding organ-specific cancer metastasis.
Murakami, T. et al. Expression of CXC chemokine receptor-4 enhances the pulmonary metastatic potential of murine B16 melanoma cells. Cancer Res. 62, 7328–7334 (2002).
Schioppa, T. et al. Regulation of the chemokine receptor CXCR4 by hypoxia. J. Exp. Med. 198, 1391–1402 (2003).
Chan, D. A. & Giaccia, A. J. Hypoxia, gene expression, and metastasis. Cancer Metastasis Rev. 26, 333–339 (2007).
Staller, P. et al. Chemokine receptor CXCR4 downregulated by von Hippel–Lindau tumour suppressor pVHL. Nature 425, 307–311 (2003).
Takanami, I. Overexpression of CCR7 mRNA in nonsmall cell lung cancer: correlation with lymph node metastasis: CCR7 expression in lung cancer. Int. J. Cancer 105, 186–189 (2003).
Günther, K. et al. Prediction of lymph node metastasis in colorectal carcinoma by expression of chemokine receptor CCR7. Int. J. Cancer 116, 726–733 (2005).
Amersi, F. F. et al. Activation of CCR9/CCL25 in cutaneous melanoma mediates preferential metastasis to the small intestine. Clin. Cancer Res. 14, 638–645 (2008).
Zlotnik, A., Burkhardt, A. M. & Homey, B. Homeostatic chemokine receptors and organ-specific metastasis. Nat. Rev. Immunol. 11, 597–606 (2011).
Spranger, S., Bao, R. & Gajewski, T. F. Melanoma-intrinsic β-catenin signalling prevents anti-tumour immunity. Nature 523, 231–235 (2015).
Peng, D. et al. Epigenetic silencing of TH1-type chemokines shapes tumour immunity and immunotherapy. Nature 527, 249–253 (2015). This study, along with Staller et al. (2003) and Spranger et al. (2015), highlights the importance of cancer genetics and epigenetics in chemokine effects on TME inflammation.
Bindea, G. et al. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity 39, 782–795 (2013).
Martin, T. D. et al. The adaptive immune system is a major driver of selection for tumor suppressor gene inactivation. Science 373, 1327–1335 (2021).
Zeng, Q. et al. Aberrant hyperexpression of the RNA binding protein FMRP in tumors mediates immune evasion. Science 378, eabl7207 (2022).
Thompson, S. et al. Regulation of chemokine function: the roles of GAG-binding and post-translational nitration. Int. J. Mol. Sci. 18, 1692 (2017).
Stone, M. J., Hayward, J. A., Huang, C., Huma, Z. E. & Sanchez, J. Mechanisms of regulation of the chemokine-receptor network. Int. J. Mol. Sci. 18, 342 (2017).
Hartmann, T. N. et al. A crosstalk between intracellular CXCR7 and CXCR4 involved in rapid CXCL12-triggered integrin activation but not in chemokine-triggered motility of human T lymphocytes and CD34 + cells. J. Leukoc. Biol. 84, 1130–1140 (2008).
Levoye, A., Balabanian, K., Baleux, F., Bachelerie, F. & Lagane, B. CXCR7 heterodimerizes with CXCR4 and regulates CXCL12-mediated G protein signaling. Blood 113, 6085–6093 (2009).
Nibbs, R. J. B. & Graham, G. J. Immune regulation by atypical chemokine receptors. Nat. Rev. Immunol. 13, 815–829 (2013).
Rot, A. Contribution of Duffy antigen to chemokine function. Cytokine Growth Factor Rev. 16, 687–694 (2005).
Addison, C. L., Belperio, J. A., Burdick, M. D. & Strieter, R. M. Overexpression of the duffy antigen receptor for chemokines (DARC) by NSCLC tumor cells results in increased tumor necrosis. BMC Cancer 4, 28 (2004).
Wang, J. et al. Enhanced expression of Duffy antigen receptor for chemokines by breast cancer cells attenuates growth and metastasis potential. Oncogene 25, 7201–7211 (2006).
Shen, H., Schuster, R., Stringer, K. F., Waltz, S. E. & Lentsch, A. B. The Duffy antigen/receptor for chemokines (DARC) regulates prostate tumor growth. FASEB J. 20, 59–64 (2006).
Horton, L. W., Yu, Y., Zaja-Milatovic, S., Strieter, R. M. & Richmond, A. Opposing roles of murine Duffy antigen receptor for chemokine and murine CXC chemokine receptor-2 receptors in murine melanoma tumor growth. Cancer Res. 67, 9791–9799 (2007).
Zeng, X.-H. et al. Coexpression of atypical chemokine binders (ACBs) in breast cancer predicts better outcomes. Breast Cancer Res. Treat. 125, 715–727 (2011).
Maeda, S. et al. Duffy antigen receptor for chemokines (DARC) expressing in cancer cells inhibits tumor progression by suppressing CXCR2 signaling in human pancreatic ductal adenocarcinoma. Cytokine 95, 12–21 (2017).
Sun, G., Wang, Y., Zhu, Y., Huang, C. & Ji, Q. Duffy antigen receptor for chemokines in laryngeal squamous cell carcinoma as a negative regulator. Acta Otolaryngol. 131, 197–203 (2011).
Latini, F. R. M. et al. DARC (Duffy) and BCAM (Lutheran) reduced expression in thyroid cancer. Blood Cells Mol. Dis. 50, 161–165 (2013).
Farnsworth, R. H., Karnezis, T., Maciburko, S. J., Mueller, S. N. & Stacker, S. A. The interplay between lymphatic vessels and chemokines. Front. Immunol. 10, 518 (2019).
Wu, F.-Y. et al. Chemokine decoy receptor D6 plays a negative role in human breast cancer. Mol. Cancer Res. 6, 1276–1288 (2008).
Nibbs, R. J. B. et al. The atypical chemokine receptor D6 suppresses the development of chemically induced skin tumors. J. Clin. Invest. 117, 1884–1892 (2007).
Vetrano, S. et al. The lymphatic system controls intestinal inflammation and inflammation-associated colon cancer through the chemokine decoy receptor D6. Gut 59, 197–206 (2010).
Zeng, X.-H. et al. Absence of multiple atypical chemokine binders (ACBs) and the presence of VEGF and MMP-9 predict axillary lymph node metastasis in early breast carcinomas. Med. Oncol. 31, 145 (2014).
Hou, T. et al. Atypical chemokine receptors predict lymph node metastasis and prognosis in patients with cervical squamous cell cancer. Gynecol. Oncol. 130, 181–187 (2013).
Hansell, C. A. H. et al. The atypical chemokine receptor Ackr2 constrains NK cell migratory activity and promotes metastasis. J. Immunol. 201, 2510–2519 (2018).
Massara, M. et al. ACKR2 in hematopoietic precursors as a checkpoint of neutrophil release and anti-metastatic activity. Nat. Commun. 9, 676 (2018).
Savino, B. et al. ERK-dependent downregulation of the atypical chemokine receptor D6 drives tumor aggressiveness in kaposi sarcoma. Cancer Immunol. Res. 2, 679–689 (2014).
Smit, M. J. et al. The CXCL12/CXCR4/ACKR3 axis in the tumor microenvironment: signaling, crosstalk, and therapeutic targeting. Annu. Rev. Pharmacol. Toxicol. 61, 541–563 (2021).
Sierro, F. et al. Disrupted cardiac development but normal hematopoiesis in mice deficient in the second CXCL12/SDF-1 receptor, CXCR7. Proc. Natl Acad. Sci. USA 104, 14759–14764 (2007).
Burns, J. M. et al. A novel chemokine receptor for SDF-1 and I-TAC involved in cell survival, cell adhesion, and tumor development. J. Exp. Med. 203, 2201–2213 (2006).
Berahovich, R. D. et al. Endothelial expression of CXCR7 and the regulation of systemic CXCL12 levels. Immunology 141, 111–122 (2014).
D’Alterio, C. et al. Concomitant CXCR4 and CXCR7 expression predicts poor prognosis in renal cancer. Curr. Cancer Drug Targets 10, 772–781 (2010).
Yao, X., Zhou, L., Han, S. & Chen, Y. High expression of CXCR4 and CXCR7 predicts poor survival in gallbladder cancer. J. Int. Med. Res. 39, 1253–1264 (2011).
D’Alterio, C. et al. A prognostic model comprising pT stage, N status, and the chemokine receptors CXCR4 and CXCR7 powerfully predicts outcome in neoadjuvant resistant rectal cancer patients: predictive model in rectal cancer patients. Int. J. Cancer 135, 379–390 (2014).
Liberman, J. et al. Involvement of the CXCR7/CXCR4/CXCL12 axis in the malignant progression of human neuroblastoma. PLoS ONE 7, e43665 (2012).
Miao, Z. et al. CXCR7 (RDC1) promotes breast and lung tumor growth in vivo and is expressed on tumor-associated vasculature. Proc. Natl Acad. Sci. USA 104, 15735–15740 (2007).
Stacer, A. C. et al. Endothelial CXCR7 regulates breast cancer metastasis. Oncogene 35, 1716–1724 (2016).
Ulvmar, M. H. et al. The atypical chemokine receptor CCRL1 shapes functional CCL21 gradients in lymph nodes. Nat. Immunol. 15, 623–630 (2014). This is a seminal study demonstrating the importance of ACKRs in chemokine gradient formation.
Bryce, S. A. et al. ACKR4 on stromal cells scavenges CCL19 to enable CCR7-dependent trafficking of APCs from inflamed skin to lymph nodes. J. Immunol. 196, 3341–3353 (2016).
Whyte, C. E. et al. ACKR4 restrains antitumor immunity by regulating CCL21. J. Exp. Med. 217, e20190634 (2020).
Peske, J. D. et al. Effector lymphocyte-induced lymph node-like vasculature enables naive T-cell entry into tumours and enhanced anti-tumour immunity. Nat. Commun. 6, 7114 (2015).
Wangmo, D. et al. ACKR4 in tumor cells regulates dendritic cell migration to tumor-draining lymph nodes and T-cell priming. Cancers 13, 5021 (2021).
Feng, L.-Y., Ou, Z.-L., Wu, F.-Y., Shen, Z.-Z. & Shao, Z.-M. Involvement of a novel chemokine decoy receptor CCX-CKR in breast cancer growth, metastasis and patient survival. Clin. Cancer Res. 15, 2962–2970 (2009).
Torphy, R. J., Yee, E. J., Schulick, R. D. & Zhu, Y. Atypical chemokine receptors: emerging therapeutic targets in cancer. Trends Pharmacol. Sci. 43, 1085–1097 (2022).
Torphy, R. J. et al. GPR182 limits antitumor immunity via chemokine scavenging in mouse melanoma models. Nat. Commun. 13, 97 (2022).
Ludwig, A. & Weber, C. Transmembrane chemokines: versatile ‘special agents’ in vascular inflammation. Thromb. Haemost. 97, 694–703 (2007).
Metzemaekers, M., Van Damme, J., Mortier, A. & Proost, P. Regulation of chemokine activity — a focus on the role of dipeptidyl peptidase IV/CD26. Front. Immunol. 7, 1–23 (2016).
Busek, P., Duke-Cohan, J. S. & Sedo, A. Does DPP-iv inhibition offer new avenues for therapeutic intervention in malignant disease? Cancers 14, 2072 (2022).
Barreira Da Silva, R. et al. Dipeptidylpeptidase 4 inhibition enhances lymphocyte trafficking, improving both naturally occurring tumor immunity and immunotherapy. Nat. Immunol. 16, 850–858 (2015).
Nishina, S. et al. Dipeptidyl peptidase 4 inhibitors reduce hepatocellular carcinoma by activating lymphocyte chemotaxis in mice. Cell. Mol. Gastroenterol. Hepatol. 7, 115–134 (2019).
Scheltens, P. et al. Safety, tolerability and efficacy of the glutaminyl cyclase inhibitor PQ912 in Alzheimer’s disease: results of a randomized, double-blind, placebo-controlled phase 2a study. Alzheimers Res. Ther. 10, 107 (2018).
Barreira da Silva, R. et al. Loss of the intracellular enzyme QPCTL limits chemokine function and reshapes myeloid infiltration to augment tumor immunity. Nat. Immunol. 23, 568–580 (2022). This paper reveals unanticipated complexity in the proteolytic regulation of chemokine activity.
Messina, J. L. et al. 12-Chemokine gene signature identifies lymph node-like structures in melanoma: potential for patient selection for immunotherapy? Sci. Rep. 2, 765 (2012).
Cristescu, R. et al. Pan-tumor genomic biomarkers for PD-1 checkpoint blockade-based immunotherapy. Science 362, eaar3593 (2018).
Wang, X., Lu, X.-L., Zhao, H.-Y., Zhang, F.-C. & Jiang, X.-B. A novel recombinant protein of IP10-EGFRvIIIscFv and CD8+ cytotoxic T lymphocytes synergistically inhibits the growth of implanted glioma in mice. Cancer Immunol. Immunother. 62, 1261–1272 (2013).
Lin, X. et al. A single-chain variable fragment antibody/chemokine fusion protein targeting human endoglin to enhance the anti-tumor activity of cytokine-induced killer cells. J. Biomed. Nanotechnol. 17, 1574–1583 (2021).
Williford, J.-M. et al. Recruitment of CD103+ dendritic cells via tumor-targeted chemokine delivery enhances efficacy of checkpoint inhibitor immunotherapy. Sci. Adv. 5, eaay1357 (2019).
Eckert, E. C. et al. Generation of a tumor-specific chemokine gradient using oncolytic vesicular stomatitis virus encoding CXCL9. Mol. Ther. Oncol. 16, 63–74 (2020).
Liu, Z. et al. CXCL11-armed oncolytic poxvirus elicits potent antitumor immunity and shows enhanced therapeutic efficacy. OncoImmunol 5, e1091554 (2016).
Adachi, K. et al. IL-7 and CCL19 expression in CAR-T cells improves immune cell infiltration and CAR-T cell survival in the tumor. Nat. Biotechnol. 36, 346–351 (2018).
Goto, S. et al. Enhanced anti-tumor efficacy of IL-7/CCL19-producing human CAR-T cells in orthotopic and patient-derived xenograft tumor models. Cancer Immunol. Immunother. 70, 2503–2515 (2021).
Pang, N. et al. IL-7 and CCL19-secreting CAR-T cell therapy for tumors with positive glypican-3 or mesothelin. J. Hematol. Oncol. 14, 118 (2021).
Luo, H. et al. Coexpression of IL7 and CCL21 increases efficacy of CAR-T cells in solid tumors without requiring preconditioned lymphodepletion. Clin. Cancer Res. 26, 5494–5505 (2020).
Pienta, K. J. et al. Phase 2 study of carlumab (CNTO 888), a human monoclonal antibody against CC-chemokine ligand 2 (CCL2), in metastatic castration-resistant prostate cancer. Invest. N. Drugs 31, 760–768 (2013).
Dominguez, C., McCampbell, K. K., David, J. M. & Palena, C. Neutralization of IL-8 decreases tumor PMN-MDSCs and reduces mesenchymalization of claudin-low triple-negative breast cancer. JCI Insight 2, e94296 (2017).
Bilusic, M. et al. Phase I trial of HuMax-IL8 (BMS-986253), an anti-IL-8 monoclonal antibody, in patients with metastatic or unresectable solid tumors. J. Immunother. Cancer 7, 240 (2019).
Kurose, K. et al. Phase Ia study of FoxP3+ CD4 treg depletion by infusion of a humanized anti-CCR4 antibody, KW-0761, in cancer patients. Clin. Cancer Res. 21, 4327–4336 (2015).
Saito, T. et al. Phase Ib study on the humanized anti-CCR4 antibody, KW-0761, in advanced solid tumors. Nagoya J. Med. Sci. 83, 827–840 (2021).
Maeda, Y. et al. Depletion of central memory CD8+ T cells might impede the antitumor therapeutic effect of mogamulizumab. Nat. Commun. 12, 7280 (2021).
Kim, Y. H. et al. Mogamulizumab versus vorinostat in previously treated cutaneous T-cell lymphoma (MAVORIC): an international, open-label, randomised, controlled phase 3 trial. Lancet Oncol. 19, 1192–1204 (2018).
Haruna, M. et al. The impact of CCR8+ regulatory T cells on cytotoxic T cell function in human lung cancer. Sci. Rep. 12, 5377 (2022).
Kidani, Y. et al. CCR8-targeted specific depletion of clonally expanded Treg cells in tumor tissues evokes potent tumor immunity with long-lasting memory. Proc. Natl Acad. Sci. USA 119, e2114282119 (2022).
Fattori, S. et al. Therapeutic targeting of tumor-infiltrating regulatory T cells in breast cancer. Cancer Res. 82, 3868–3879 (2022).
Thomas, S. K. et al. Phase I study of an active immunotherapy for asymptomatic phase lymphoplasmacytic lymphoma with DNA vaccines encoding antigen-chemokine fusion: study protocol. BMC Cancer 18, 187 (2018).
Hillemanns, P. et al. A therapeutic antigen-presenting cell-targeting DNA vaccine VB10.16 in HPV16-positive high-grade cervical intraepithelial neoplasia: results from a phase I/IIa trial. Clin. Cancer Res. 28, 4885–4892 (2022).
Jin, L. et al. CXCR1- or CXCR2-modified CAR T cells co-opt IL-8 for maximal antitumor efficacy in solid tumors. Nat. Commun. 10, 4016 (2019).
Idorn, M. et al. Chemokine receptor engineering of T cells with CXCR2 improves homing towards subcutaneous human melanomas in xenograft mouse model. OncoImmunol 7, e1450715 (2018).
Peng, W. et al. Transduction of tumor-specific T cells with CXCR2 chemokine receptor improves migration to tumor and antitumor immune responses. Clin. Cancer Res. 16, 5458–5468 (2010).
Brown, C. E. et al. Tumor-derived chemokine MCP-1/CCL2 is sufficient for mediating tumor tropism of adoptively transferred T cells. J. Immunol. 179, 3332–3341 (2007).
Craddock, J. A. et al. Enhanced tumor trafficking of GD2 chimeric antigen receptor T cells by expression of the chemokine receptor CCR2b. J. Immunother. 33, 780–788 (2010).
Moon, E. K. et al. Expression of a functional CCR2 receptor enhances tumor localization and tumor eradication by retargeted human T cells expressing a mesothelin-specific chimeric antibody receptor. Clin. Cancer Res. 17, 4719–4730 (2011).
Lesch, S. et al. T cells armed with C-X-C chemokine receptor type 6 enhance adoptive cell therapy for pancreatic tumours. Nat. Biomed. Eng. 5, 1246–1260 (2021).
Cadilha, B. L. et al. Combined tumor-directed recruitment and protection from immune suppression enable CAR T cell efficacy in solid tumors. Sci. Adv. 7, eabi5781 (2021).
Worthington, J. J. et al. Integrin αvβ8-mediated TGF-β activation by effector regulatory T cells is essential for suppression of T-cell-mediated inflammation. Immunity 42, 903–915 (2015).
Foeng, J., Comerford, I. & McColl, S. R. Harnessing the chemokine system to home CAR-T cells into solid tumors. Cell Rep. Med. 3, 100543 (2022).
Märkl, F., Huynh, D., Endres, S. & Kobold, S. Utilizing chemokines in cancer immunotherapy. Trends Cancer https://doi.org/10.1016/j.trecan.2022.04.001 (2022).
Poeta, V. M., Massara, M., Capucetti, A. & Bonecchi, R. Chemokines and chemokine receptors: new targets for cancer immunotherapy. Front. Immunol. 10, 1–10 (2019).
Ortiz Zacarías, N. V., Bemelmans, M. P., Handel, T. M., De Visser, K. E. & Heitman, L. H. Anticancer opportunities at every stage of chemokine function. Trends Pharmacol. Sci. 42, 912–928 (2021).
Elhanani, O., Ben-Uri, R. & Keren, L. Spatial profiling technologies illuminate the tumor microenvironment. Cancer Cell 41, 404–420 (2023).
Moses, L. & Pachter, L. Museum of spatial transcriptomics. Nat. Methods 19, 534–546 (2022).
Mujal, A. M. & Krummel, M. F. Immunity as a continuum of archetypes. Science 364, 28–29 (2019).
DeVries, M. E. et al. Defining the origins and evolution of the chemokine/chemokine receptor system. J. Immunol. 176, 401–415 (2006). This study is an interesting perspective on the origin of the chemokine system.
Mouse Genome Sequencing Consortium. Initial sequencing and comparative analysis of the mouse genome. Nature 420, 520–562 (2002).
Berger, E. A., Murphy, P. M. & Farber, J. M. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu. Rev. Immunol. 17, 657–700 (1999).
Pontejo, S. M. & Murphy, P. M. Chemokines encoded by herpesviruses. J. Leukoc. Biol. 102, 1199–1217 (2017).
Fang, W. B. et al. CCL2/CCR2 chemokine signaling coordinates survival and motility of breast cancer cells through smad3 protein- and p42/44 mitogen-activated protein kinase (MAPK)-dependent mechanisms. J. Biol. Chem. 287, 36593–36608 (2012).
Acevedo, D. S. et al. Regulation of growth, invasion and metabolism of breast ductal carcinoma through CCL2/CCR2 signaling interactions with MET receptor tyrosine kinases. Neoplasia 28, 100791 (2022).
Tsuyada, A. et al. CCL2 mediates cross-talk between cancer cells and stromal fibroblasts that regulates breast cancer stem cells. Cancer Res. 72, 2768–2779 (2012).
Kodama, T. et al. CCL3–CCR5 axis contributes to progression of esophageal squamous cell carcinoma by promoting cell migration and invasion via Akt and ERK pathways. Lab. Invest. 100, 1140–1157 (2020).
Hosono, M. et al. CXCL8 derived from tumor-associated macrophages and esophageal squamous cell carcinomas contributes to tumor progression by promoting migration and invasion of cancer cells. Oncotarget 8, 106071–106088 (2017).
Wang, N. et al. CXCL1 derived from tumor-associated macrophages promotes breast cancer metastasis via activating NF-κB/SOX4 signaling. Cell Death Dis. 9, 880 (2018).
Raman, D., Baugher, P. J., Thu, Y. M. & Richmond, A. Role of chemokines in tumor growth. Cancer Lett. 256, 137–165 (2007).
Fein, M. R. et al. Cancer cell CCR2 orchestrates suppression of the adaptive immune response. J. Exp. Med. 217, e20181551 (2020). This study demonstrates the in vivo importance of cancer cell expression of chemokine receptors.
Acknowledgements
This work was supported in part by NIH grants P01-CA240239, R01 AI163223 and R01 AI123349 (to T.R.M.).
Author information
Authors and Affiliations
Contributions
All authors researched data for the article and contributed substantially to discussion of the content. L.M.A. and T.R.M. wrote the article. T.R.M. reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Cancer thanks Frances Balkwill who co-reviewed with Beatrice Malacrida, Raffaella Bonecchi and Sebastian Kobold for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Antibody-dependent cellular cytotoxicity
-
(ADCC). A mechanism through which Fc receptor-expressing immune effector cells, such as NK cells or macrophages, can recognize and kill antibody-coated target cells.
- B follicles
-
Large, well-organized clusters of B cells attracted by CXCL13-expressing mesenchymal cells called follicular dendritic cells and found in secondary lymphoid tissues and tertiary lymphoid structures.
- Citrullination
-
A post-translational protein modification whereby arginine residues are enzymatically converted to citrulline, which can trigger autoimmune reactions against citrullinated proteins, but has also been suggested to alter the function or activity of some proteins, including chemokines.
- Cytokine-induced killer cells
-
A heterogenous population of cells with NK and T cell features generated by ex vivo activation and treatment of peripheral blood mononuclear cells of a patient with cytokines including IL-2, IL-1 and IFNγ.
- Diapedesis
-
The migration of blood-borne leukocytes through the blood vessel wall via interendothelial junctions (paracellular) or through endothelial cells (transcellular) into the surrounding tissue.
- Fibroblastic reticular cells
-
(FRCs). Specialized populations of fibroblast-like mesenchymal cells that provide structure and dynamically organize functional compartments in lymphoid tissues through their interactions with immune cells.
- Germinal centre dark zone
-
The part of the germinal centre where B cells proliferate and hypermutate their B cell receptor genes before again migrating through the light zone, where they are selected for their antigen affinity.
- Granulopoiesis
-
The part of haematopoiesis that leads to the production of neutrophils, eosinophils and basophils.
- Haptotactic gradients
-
Cell motility directed by gradients of immobilized guidance molecules, such as ECM-bound chemokines or adhesion molecules, unlike chemotaxis, where cells follow guidance molecules in solution.
- Isotype switching
-
A genetic mechanism in B cells that changes the immunoglobulin constant region to alter antibody effector function and class, for example, from IgM to IgG.
- Leukotrienes
-
Inflammatory lipid signal molecules produced by white blood cells through oxidative metabolism of the 20-base polymer/oligonucleotide arachidonic acid in their membranes.
- Lymph node anlagen
-
The incipient structures of lymph node organogenesis during embryonic development.
- Lymph node interfollicular areas
-
The lymph node areas between B follicles through which dendritic cells enter lymph nodes and which are occupied by high densities of innate lymphocytes, γδT cells and NKT cells.
- Lymph node subcapsular sinus
-
The lymphatic endothelium-lined space between lymph node capsule and cortex into which afferent lymph and lymph-borne solutes, particles and cells are collected.
- Lymphoid-tissue inducer (LTi) cells
-
A lymphotoxin-producing innate lymphocyte population that stimulates mesenchymal cells to express chemokines and adhesion molecules to promote the formation and expansion of lymph node anlagen.
- Lymphoid-tissue organizer (LTo) cells
-
The lymphotoxin-induced mesenchymal precursors of lymphoid-tissue FRC in lymph node anlagen and other primitive lymphoid tissues.
- Necroptosis
-
A caspase-independent, pro-inflammatory form of programmed cell death.
- Neutrophil extracellular traps
-
(NETs). Fibril matrices composed of chromatin and granule proteins released by neutrophils to entrap and kill extracellular pathogens.
- Peyer’s patches
-
Secondary lymphoid tissues in the antimesenteric wall of the small intestine composed of organized clusters of subepithelial lymphoid follicles.
- Single-chain variable fragment
-
(scFv). Recombinant fusion protein of the light and heavy chain variable regions of an antibody, connected via a linker but lacking antibody constant regions.
- Tertiary lymphoid structures
-
(TLSs). Organized lymphoid tissues that are not developmentally programmed, but arise at sites of chronic inflammation, including tumour microenvironments.
- Trans-endocytosis
-
A molecular process whereby cells use cell surface receptors to bind and remove surface proteins on cells they interact with for internalization and degradation of those target proteins.
- Trans-presentation
-
The mechanism by which cells expressing IL-15, alternative to releasing it as a soluble factor, can express and use the IL-15Rα chain to present this cytokine to cells expressing the IL-15Rβ/γC signalling receptor chains.
- Vitiligo
-
A depigmenting autoimmune disease mediated by T cells that recognize antigens in the melanin synthesis pathway, and a common side effect of effective anti-melanoma immunity.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mempel, T.R., Lill, J.K. & Altenburger, L.M. How chemokines organize the tumour microenvironment. Nat Rev Cancer 24, 28–50 (2024). https://doi.org/10.1038/s41568-023-00635-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41568-023-00635-w