Abstract
The recently uncovered key role of the peripheral and central nervous systems in controlling tumorigenesis and metastasis has opened a new area of research to identify innovative approaches against cancer. Although the 'neural addiction' of cancer is only partially understood, in this Perspective we discuss the current knowledge and perspectives on peripheral and central nerve circuitries and brain areas that can support tumorigenesis and metastasis and the possible reciprocal influence that the brain and peripheral tumours exert on one another. Tumours can build up local autonomic and sensory nerve networks and are able to develop a long-distance relationship with the brain through circulating adipokines, inflammatory cytokines, neurotrophic factors or afferent nerve inputs, to promote cancer initiation, growth and dissemination. In turn, the central nervous system can affect tumour development and metastasis through the activation or dysregulation of specific central neural areas or circuits, as well as neuroendocrine, neuroimmune or neurovascular systems. Studying neural circuitries in the brain and tumours, as well as understanding how the brain communicates with the tumour or how intratumour nerves interplay with the tumour microenvironment, can reveal unrecognized mechanisms that promote cancer development and progression and open up opportunities for the development of novel therapeutic strategies. Targeting the dysregulated peripheral and central nervous systems might represent a novel strategy for next-generation cancer treatment that could, in part, be achieved through the repurposing of neuropsychiatric drugs in oncology.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Monje, M. et al. Roadmap for the emerging field of cancer neuroscience. Cell 181, 219–222 (2020).
Boilly, B., Faulkner, S., Jobling, P. & Hondermarck, H. Nerve dependence: from regeneration to cancer. Cancer Cell 31, 342–354 (2017).
Hajdu, S. I. Greco-Roman thought about cancer. Cancer 100, 2048–2051 (2004).
Faguet, G. B. A brief history of cancer: age-old milestones underlying our current knowledge database. Int. J. Cancer 136, 2022–2036 (2015).
Bissell, M. J. & Hines, W. C. Why don’t we get more cancer? A proposed role of the microenvironment in restraining cancer progression. Nat. Med. 17, 320–329 (2011).
Paget, S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev. 8, 98–101 (1989).
Folkman, J. Tumor angiogenesis: therapeutic implications. N. Engl. J. Med. 285, 1182–1186 (1971).
Liebig, C., Ayala, G., Wilks, J. A., Berger, D. H. & Albo, D. Perineural invasion in cancer: a review of the literature. Cancer 115, 3379–3391 (2009).
Amit, M., Na’ara, S. & Gil, Z. Mechanisms of cancer dissemination along nerves. Nat. Rev. Cancer 16, 399–408 (2016).
Cohen, S., Levi-Montalcini, R. & Hamburger, V. A nerve growth-stimulating factor isolated from sarcomas 37 and 180. Proc. Natl Acad. Sci. USA 40, 1014–1018 (1954).
Adriaenssens, E. et al. Nerve growth factor is a potential therapeutic target in breast cancer. Cancer Res. 68, 346–351 (2008).
Levi-Montalcini, R., Meyer, H. & Hamburger, V. In vitro experiments on the effects of mouse sarcomas 180 and 37 on the spinal and sympathetic ganglia of the chick embryo. Cancer Res. 14, 49–57 (1954).
Saul, A. N. et al. Chronic stress and susceptibility to skin cancer. J. Natl Cancer Inst. 97, 1760–1767 (2005).
Reiche, E. M., Nunes, S. O. & Morimoto, H. K. Stress, depression, the immune system, and cancer. Lancet Oncol. 5, 617–625 (2004).
Riley, V. Mouse mammary tumors: alteration of incidence as apparent function of stress. Science 189, 465–467 (1975).
Visintainer, M. A., Volpicelli, J. R. & Seligman, M. E. Tumor rejection in rats after inescapable or escapable shock. Science 216, 437–439 (1982).
Sklar, L. S. & Anisman, H. Stress and coping factors influence tumor growth. Science 205, 513–515 (1979).
Magnon, C. et al. Autonomic nerve development contributes to prostate cancer progression. Science 341, 1236361 (2013).
Zhao, C. M. et al. Denervation suppresses gastric tumorigenesis. Sci. Transl Med. 6, 250ra115 (2014).
Hayakawa, Y. et al. Nerve growth factor promotes gastric tumorigenesis through aberrant cholinergic signaling. Cancer Cell 31, 21–34 (2017).
Peterson, S. C. et al. Basal cell carcinoma preferentially arises from stem cells within hair follicle and mechanosensory niches. Cell Stem Cell 16, 400–412 (2015).
Stopczynski, R. E. et al. Neuroplastic changes occur early in the development of pancreatic ductal adenocarcinoma. Cancer Res. 74, 1718–1727 (2014).
Saloman, J. L. et al. Ablation of sensory neurons in a genetic model of pancreatic ductal adenocarcinoma slows initiation and progression of cancer. Proc. Natl Acad. Sci. USA 113, 3078–3083 (2016).
Renz, B. W. et al. β2 Adrenergic-neurotrophin feedforward loop promotes pancreatic cancer. Cancer Cell 34, 863–867 (2018).
Banh, R. S. et al. Neurons release serine to support mRNA translation in pancreatic cancer. Cell 183, 1202–1218 (2020).
Pundavela, J. et al. Nerve fibers infiltrate the tumor microenvironment and are associated with nerve growth factor production and lymph node invasion in breast cancer. Mol. Oncol. 9, 1626–1635 (2015).
Kamiya, A. et al. Genetic manipulation of autonomic nerve fiber innervation and activity and its effect on breast cancer progression. Nat. Neurosci. 22, 1289–1305 (2019).
Gysler, S. M. & Drapkin, R. Tumor innervation: peripheral nerves take control of the tumor microenvironment. J. Clin. Investig. https://doi.org/10.1172/JCI147276 (2021).
Silverman, D. A. et al. Cancer-associated neurogenesis and nerve-cancer cross-talk. Cancer Res. 81, 1431–1440 (2021).
Zahalka, A. H. & Frenette, P. S. Nerves in cancer. Nat. Rev. Cancer 20, 143–157 (2020).
Magnon, C. The adrenergic nerve network in cancer. Adv. Exp. Med. Biol. 1329, 271–294 (2021).
McEwen, B. S. Stress, adaptation, and disease. Allostasis and allostatic load. Ann. NY Acad. Sci. 840, 33–44 (1998).
Obradovic, M. M. S. et al. Glucocorticoids promote breast cancer metastasis. Nature 567, 540–544 (2019).
Herman, J. P. et al. Regulation of the hypothalamic-pituitary-adrenocortical stress response. Compr. Physiol. 6, 603–621 (2016).
Gallo-Payet, N., Martinez, A. & Lacroix, A. Editorial: ACTH action in the adrenal cortex: from molecular biology to pathophysiology. Front. Endocrinol. 8, 101 (2017).
Cryer, P. E. Physiology and pathophysiology of the human sympathoadrenal neuroendocrine system. N. Engl. J. Med. 303, 436–444 (1980).
Thaker, P. H. et al. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat. Med. 12, 939–944 (2006).
Kvetnansky, R. et al. Sympathoadrenal system in stress. Interaction with the hypothalamic-pituitary-adrenocortical system. Ann. NY Acad. Sci. 771, 131–158 (1995).
Ehrhart-Bornstein, M. & Bornstein, S. R. Cross-talk between adrenal medulla and adrenal cortex in stress. Ann. NY Acad. Sci. 1148, 112–117 (2008).
Zuckerman-Levin, N., Tiosano, D., Eisenhofer, G., Bornstein, S. & Hochberg, Z. The importance of adrenocortical glucocorticoids for adrenomedullary and physiological response to stress: a study in isolated glucocorticoid deficiency. J. Clin. Endocrinol. Metab. 86, 5920–5924 (2001).
Cunningham, E. T. Jr & Sawchenko, P. E. Anatomical specificity of noradrenergic inputs to the paraventricular and supraoptic nuclei of the rat hypothalamus. J. Comp. Neurol. 274, 60–76 (1988).
Herman, J. P. Regulation of hypothalamo-pituitary-adrenocortical responses to stressors by the nucleus of the solitary tract/dorsal vagal complex. Cell Mol. Neurobiol. 38, 25–35 (2018).
Peters, L. J. & Kelly, H. The influence of stress and stress hormones on the transplantability of a non-immunogenic syngeneic murine tumor. Cancer 39, 1482–1488 (1977).
Sigurdsson, T. & Duvarci, S. Hippocampal-prefrontal interactions in cognition, behavior and psychiatric disease. Front. Syst. Neurosci. 9, 190 (2015).
Schagen, S. B. et al. Monitoring and optimising cognitive function in cancer patients: present knowledge and future directions. EJC Suppl. 12, 29–40 (2014).
Ahles, T. A. & Root, J. C. Cognitive effects of cancer and cancer treatments. Annu. Rev. Clin. Psychol. 14, 425–451 (2018).
Ahles, T. A. et al. Cognitive function in breast cancer patients prior to adjuvant treatment. Breast Cancer Res. Treat. 110, 143–152 (2008).
Vardy, J. L. et al. Cognitive function in patients with colorectal cancer who do and do not receive chemotherapy: a prospective, longitudinal, controlled study. J. Clin. Oncol. 33, 4085–4092 (2015).
Mandelblatt, J. S. et al. Cognitive impairment in older patients with breast cancer before systemic therapy: is there an interaction between cancer and comorbidity? J. Clin. Oncol. 32, 1909–1918 (2014).
Lange, M. et al. Baseline cognitive functions among elderly patients with localised breast cancer. Eur. J. Cancer 50, 2181–2189 (2014).
Bergouignan, L. et al. Breast cancer affects both the hippocampus volume and the episodic autobiographical memory retrieval. PLoS ONE 6, e25349 (2011).
Morel, N. et al. Emotional specificities of autobiographical memory after breast cancer diagnosis. Conscious. Cogn. 35, 42–52 (2015).
Liston, C. et al. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J. Neurosci. 26, 7870–7874 (2006).
Vyas, A., Mitra, R., Shankaranarayana Rao, B. S. & Chattarji, S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J. Neurosci. 22, 6810–6818 (2002).
Murmu, M. S. et al. Changes of spine density and dendritic complexity in the prefrontal cortex in offspring of mothers exposed to stress during pregnancy. Eur. J. Neurosci. 24, 1477–1487 (2006).
Magarinos, A. M. & McEwen, B. S. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: involvement of glucocorticoid secretion and excitatory amino acid receptors. Neuroscience 69, 89–98 (1995).
Gould, E., McEwen, B. S., Tanapat, P., Galea, L. A. & Fuchs, E. Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. J. Neurosci. 17, 2492–2498 (1997).
Mitra, R., Jadhav, S., McEwen, B. S., Vyas, A. & Chattarji, S. Stress duration modulates the spatiotemporal patterns of spine formation in the basolateral amygdala. Proc. Natl Acad. Sci. USA 102, 9371–9376 (2005).
Cerqueira, J. J. et al. Morphological correlates of corticosteroid-induced changes in prefrontal cortex-dependent behaviors. J. Neurosci. 25, 7792–7800 (2005).
Liston, C., McEwen, B. S. & Casey, B. J. Psychosocial stress reversibly disrupts prefrontal processing and attentional control. Proc. Natl Acad. Sci. USA 106, 912–917 (2009).
Moghaddam, B. Bringing order to the glutamate chaos in schizophrenia. Neuron 40, 881–884 (2003).
Li, C. T., Yang, K. C. & Lin, W. C. Glutamatergic dysfunction and glutamatergic compounds for major psychiatric disorders: evidence from clinical neuroimaging studies. Front. Psychiatry 9, 767 (2018).
Schoonover, K. E., Dienel, S. J. & Lewis, D. A. Prefrontal cortical alterations of glutamate and GABA neurotransmission in schizophrenia: insights for rational biomarker development. Biomark. Neuropsychiatry https://doi.org/10.1016/j.bionps.2020.100015 (2020).
Lupien, S. J., McEwen, B. S., Gunnar, M. R. & Heim, C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat. Rev. Neurosci. 10, 434–445 (2009).
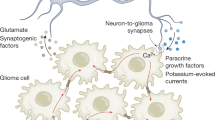
Venkatesh, H. S. et al. Electrical and synaptic integration of glioma into neural circuits. Nature 573, 539–545 (2019).
Venkataramani, V. et al. Glutamatergic synaptic input to glioma cells drives brain tumour progression. Nature 573, 532–538 (2019).
Zeng, Q. et al. Synaptic proximity enables NMDAR signalling to promote brain metastasis. Nature 573, 526–531 (2019).
Rzeski, W., Turski, L. & Ikonomidou, C. Glutamate antagonists limit tumor growth. Proc. Natl Acad. Sci. USA 98, 6372–6377 (2001).
Martirosian, V. et al. Medulloblastoma uses GABA transaminase to survive in the cerebrospinal fluid microenvironment and promote leptomeningeal dissemination. Cell Rep. 36, 109475 (2021).
Klemm, F. et al. Compensatory CSF2-driven macrophage activation promotes adaptive resistance to CSF1R inhibition in breast-to-brain metastasis. Nat. Cancer 2, 1086–1101 (2021).
Musazzi, L. et al. Acute stress increases depolarization-evoked glutamate release in the rat prefrontal/frontal cortex: the dampening action of antidepressants. PLoS ONE 5, e8566 (2010).
Popoli, M., Yan, Z., McEwen, B. S. & Sanacora, G. The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nat. Rev. Neurosci. 13, 22–37 (2011).
Munson, J. M. et al. Anti-invasive adjuvant therapy with imipramine blue enhances chemotherapeutic efficacy against glioma. Sci. Transl Med. 4, 127ra136 (2012).
Rajamanickam, S. et al. Inhibition of FoxM1-mediated DNA repair by imipramine blue suppresses breast cancer growth and metastasis. Clin. Cancer Res. 22, 3524–3536 (2016).
Buijs, R. M. & Kalsbeek, A. Hypothalamic integration of central and peripheral clocks. Nat. Rev. Neurosci. 2, 521–526 (2001).
Moore, R. Y. & Eichler, V. B. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res. 42, 201–206 (1972).
Stephan, F. K. & Zucker, I. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc. Natl Acad. Sci. USA 69, 1583–1586 (1972).
Shafi, A. A. & Knudsen, K. E. Cancer and the circadian clock. Cancer Res. 79, 3806–3814 (2019).
Lee, Y. et al. G1/S cell cycle regulators mediate effects of circadian dysregulation on tumor growth and provide targets for timed anticancer treatment. PLoS Biol. 17, e3000228 (2019).
Shilts, J., Chen, G. & Hughey, J. J. Evidence for widespread dysregulation of circadian clock progression in human cancer. PeerJ 6, e4327 (2018).
Papagiannakopoulos, T. et al. Circadian rhythm disruption promotes lung tumorigenesis. Cell Metab. 24, 324–331 (2016).
Van Dycke, K. C. et al. Chronically alternating light cycles increase breast cancer risk in mice. Curr. Biol. 25, 1932–1937 (2015).
Davis, S., Mirick, D. K. & Stevens, R. G. Night shift work, light at night, and risk of breast cancer. J. Natl Cancer Inst. 93, 1557–1562 (2001).
Cadenas, C. et al. Loss of circadian clock gene expression is associated with tumor progression in breast cancer. Cell Cycle 13, 3282–3291 (2014).
Stevens, R. G. Circadian disruption and breast cancer: from melatonin to clock genes. Epidemiology 16, 254–258 (2005).
Conlon, M., Lightfoot, N. & Kreiger, N. Rotating shift work and risk of prostate cancer. Epidemiology 18, 182–183 (2007).
Wendeu-Foyet, M. G. & Menegaux, F. Circadian disruption and prostate cancer risk: an updated review of epidemiological evidences. Cancer Epidemiol. Biomark. Prev. 26, 985–991 (2017).
Papantoniou, K. et al. Rotating night shift work and colorectal cancer risk in the nurses’ health studies. Int. J. Cancer 143, 2709–2717 (2018).
O’Neill, J. S., Maywood, E. S., Chesham, J. E., Takahashi, J. S. & Hastings, M. H. cAMP-dependent signaling as a core component of the mammalian circadian pacemaker. Science 320, 949–953 (2008).
Ueyama, T. et al. Suprachiasmatic nucleus: a central autonomic clock. Nat. Neurosci. 2, 1051–1053 (1999).
Buijs, R. M., Chun, S. J., Niijima, A., Romijn, H. J. & Nagai, K. Parasympathetic and sympathetic control of the pancreas: a role for the suprachiasmatic nucleus and other hypothalamic centers that are involved in the regulation of food intake. J. Comp. Neurol. 431, 405–423 (2001).
Fu, L., Pelicano, H., Liu, J., Huang, P. & Lee, C. The circadian gene Period2 plays an important role in tumor suppression and DNA damage response in vivo. Cell 111, 41–50 (2002).
Lee, S., Donehower, L. A., Herron, A. J., Moore, D. D. & Fu, L. Disrupting circadian homeostasis of sympathetic signaling promotes tumor development in mice. PLoS ONE 5, e10995 (2010).
Moore, R. Y., Halaris, A. E. & Jones, B. E. Serotonin neurons of the midbrain raphe: ascending projections. J. Comp. Neurol. 180, 417–438 (1978).
Jiang, Z. G., Teshima, K., Yang, Y., Yoshioka, T. & Allen, C. N. Pre- and postsynaptic actions of serotonin on rat suprachiasmatic nucleus neurons. Brain Res. 866, 247–256 (2000).
Edgar, D. M., Miller, J. D., Prosser, R. A., Dean, R. R. & Dement, W. C. Serotonin and the mammalian circadian system: II. Phase-shifting rat behavioral rhythms with serotonergic agonists. J. Biol. Rhythm. 8, 17–31 (1993).
Rea, M. A., Barrera, J., Glass, J. D. & Gannon, R. L. Serotonergic potentiation of photic phase shifts of the circadian activity rhythm. Neuroreport 6, 1417–1420 (1995).
Kalsbeek, A., Cutrera, R. A., Van Heerikhuize, J. J., Van Der Vliet, J. & Buijs, R. M. GABA release from suprachiasmatic nucleus terminals is necessary for the light-induced inhibition of nocturnal melatonin release in the rat. Neuroscience 91, 453–461 (1999).
Kalsbeek, A. et al. Melatonin sees the light: blocking GABA-ergic transmission in the paraventricular nucleus induces daytime secretion of melatonin. Eur. J. Neurosci. 12, 3146–3154 (2000).
Brennan, R., Jan, J. E. & Lyons, C. J. Light, dark, and melatonin: emerging evidence for the importance of melatonin in ocular physiology. Eye 21, 901–908 (2007).
Teclemariam-Mesbah, R., Ter Horst, G. J., Postema, F., Wortel, J. & Buijs, R. M. Anatomical demonstration of the suprachiasmatic nucleus-pineal pathway. J. Comp. Neurol. 406, 171–182 (1999).
Carrillo-Vico, A., Lardone, P. J., Alvarez-Sanchez, N., Rodriguez-Rodriguez, A. & Guerrero, J. M. Melatonin: buffering the immune system. Int. J. Mol. Sci. 14, 8638–8683 (2013).
Calvo, J. R., González-Yanes, C. & Maldonado, M. D. The role of melatonin in the cells of the innate immunity: a review. J. Pineal Res. 55, 103–120 (2013).
Glickman, G., Levin, R. & Brainard, G. C. Ocular input for human melatonin regulation: relevance to breast cancer. Neuro Endocrinol. Lett. 23, 17–22 (2002).
Lissoni, P. et al. A clinical study of the pineal gland activity in oncologic patients. Cancer 57, 837–842 (1986).
Blask, D. E. et al. Melatonin-depleted blood from premenopausal women exposed to light at night stimulates growth of human breast cancer xenografts in nude rats. Cancer Res. 65, 11174–11184 (2005).
Straif, K. et al. Carcinogenicity of shift-work, painting, and fire-fighting. Lancet Oncol. 8, 1065–1066 (2007).
IARC Monographs Vol 124 Group. Carcinogenicity of night shift work. Lancet Oncol. 20, 1058–1059 (2019).
Blask, D. E., Dauchy, R. T. & Sauer, L. A. Putting cancer to sleep at night: the neuroendocrine/circadian melatonin signal. Endocrine 27, 179–188 (2005).
Tarocco, A. et al. Melatonin as a master regulator of cell death and inflammation: molecular mechanisms and clinical implications for newborn care. Cell Death Dis. 10, 317 (2019).
Mao, L. et al. Melatonin suppression of aerobic glycolysis (Warburg effect), survival signalling and metastasis in human leiomyosarcoma. J. Pineal Res. 60, 167–177 (2016).
Xiang, S. et al. Doxorubicin resistance in breast cancer is driven by light at night-induced disruption of the circadian melatonin signal. J. Pineal Res. 59, 60–69 (2015).
Colwell, C. S., Foster, R. G. & Menaker, M. NMDA receptor antagonists block the effects of light on circadian behavior in the mouse. Brain Res. 554, 105–110 (1991).
Ding, J. M. et al. Resetting the biological clock: mediation of nocturnal circadian shifts by glutamate and NO. Science 266, 1713–1717 (1994).
Abe, H., Rusak, B. & Robertson, H. A. NMDA and non-NMDA receptor antagonists inhibit photic induction of Fos protein in the hamster suprachiasmatic nucleus. Brain Res. Bull. 28, 831–835 (1992).
Colwell, C. S. & Menaker, M. NMDA as well as non-NMDA receptor antagonists can prevent the phase-shifting effects of light on the circadian system of the golden hamster. J. Biol. Rhythm. 7, 125–136 (1992).
Rea, M. A., Glass, J. D. & Colwell, C. S. Serotonin modulates photic responses in the hamster suprachiasmatic nuclei. J. Neurosci. 14, 3635–3642 (1994).
Dakir, E.-H. et al. The anti-psychotic drug pimozide is a novel chemotherapeutic for breast cancer. Oncotarget 9, 34889–34910 (2018).
Chen, J. J. et al. The neuroleptic drug pimozide inhibits stem-like cell maintenance and tumorigenicity in hepatocellular carcinoma. Oncotarget 8, 17593–17609 (2017).
Chen, J. J., Zhang, L. N., Cai, N., Zhang, Z. & Ji, K. Antipsychotic agent pimozide promotes reversible proliferative suppression by inducing cellular quiescence in liver cancer. Oncol. Rep. 42, 1101–1109 (2019).
Zhou, W. et al. The antipsychotic drug pimozide inhibits cell growth in prostate cancer through suppression of STAT3 activation. Int. J. Oncol. 48, 322–328 (2016).
Mohammed, T. A. et al. A pilot phase II study of valproic acid for treatment of low-grade neuroendocrine carcinoma. Oncologist 16, 835–843 (2011).
Caponigro, F. et al. Phase II clinical study of valproic acid plus cisplatin and cetuximab in recurrent and/or metastatic squamous cell carcinoma of Head and Neck-V-CHANCE trial. BMC Cancer 16, 918 (2016).
Kao, C. H. et al. Relationship of zolpidem and cancer risk: a Taiwanese population-based cohort study. Mayo Clin. Proc. 87, 430–436 (2012).
Bonnavion, P., Jackson, A. C., Carter, M. E. & de Lecea, L. Antagonistic interplay between hypocretin and leptin in the lateral hypothalamus regulates stress responses. Nat. Commun. 6, 6266 (2015).
Fakhoury, M., Salman, I., Najjar, W., Merhej, G. & Lawand, N. The lateral hypothalamus: an uncharted territory for processing peripheral neurogenic inflammation. Front. Neurosci. 14, 101 (2020).
de Lecea, L. et al. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc. Natl Acad. Sci. USA 95, 322–327 (1998).
Sakurai, T. et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 92, 573–585 (1998).
McAlpine, C. S. et al. Sleep modulates haematopoiesis and protects against atherosclerosis. Nature 566, 383–387 (2019).
Verkasalo, P. K. et al. Sleep duration and breast cancer: a prospective cohort study. Cancer Res. 65, 9595–9600 (2005).
Gallicchio, L. & Kalesan, B. Sleep duration and mortality: a systematic review and meta-analysis. J. Sleep Res. 18, 148–158 (2009).
Kakizaki, M. et al. Sleep duration and the risk of breast cancer: the Ohsaki Cohort Study. Br. J. Cancer 99, 1502–1505 (2008).
Thompson, C. L. et al. Short duration of sleep increases risk of colorectal adenoma. Cancer 117, 841–847 (2011).
Hakim, F. et al. Fragmented sleep accelerates tumor growth and progression through recruitment of tumor-associated macrophages and TLR4 signaling. Cancer Res. 74, 1329–1337 (2014).
Borniger, J. C. et al. A Role for hypocretin/orexin in metabolic and sleep abnormalities in a mouse model of non-metastatic breast cancer. Cell Metab. 28, 118–129.e5 (2018).
Bennett, T., Bray, D. & Neville, M. W. Suvorexant, a dual orexin receptor antagonist for the management of insomnia. P T 39, 264–266 (2014).
Dayot, S. et al. In vitro, in vivo and ex vivo demonstration of the antitumoral role of hypocretin-1/orexin-A and almorexant in pancreatic ductal adenocarcinoma. Oncotarget 9, 6952–6967 (2018).
Simon, R. H., Lovett, E. J. III, Tomaszek, D. & Lundy, J. Electrical stimulation of the midbrain mediates metastatic tumor growth. Science 209, 1132–1133 (1980).
Lundy, J., Lovett, E. J. III & Conran, P. Pulmonary metastases, a potential biologic consequence of anesthetic-induced immunosuppression by thiopental. Surgery 82, 254–256 (1977).
Peraino, C., Fry, R. J. & Staffeldt, E. Brief communication: enhancement of spontaneous hepatic tumorigenesis in C3H mice by dietary phenobarbital. J. Natl Cancer Inst. 51, 1349–1350 (1973).
Peraino, C., Fry, R. J. & Staffeldt, E. Effects of varying the onset and duration of exposure to phenobarbital on its enhancement of 2-acetylaminofluorene-induced hepatic tumorigenesis. Cancer Res. 37, 3623–3627 (1977).
Morales, M. & Margolis, E. B. Ventral tegmental area: cellular heterogeneity, connectivity and behaviour. Nat. Rev. Neurosci. 18, 73–85 (2017).
Kahn, R. S. et al. Schizophrenia. Nat. Rev. Dis. Prim. 1, 15067 (2015).
Howard, L. M. et al. Cancer diagnosis in people with severe mental illness: practical and ethical issues. Lancet Oncol. 11, 797–804 (2010).
Solmi, M. et al. Disparities in cancer screening in people with mental illness across the world versus the general population: prevalence and comparative meta-analysis including 4 717 839 people. Lancet Psychiatry 7, 52–63 (2020).
Teunis, M. A. et al. Reduced tumor growth, experimental metastasis formation, and angiogenesis in rats with a hyperreactive dopaminergic system. FASEB J. 16, 1465–1467 (2002).
Peters, M. A. et al. Dopamine and serotonin regulate tumor behavior by affecting angiogenesis. Drug Resist. Updat. 17, 96–104 (2014).
Ben-Shaanan, T. L. et al. Modulation of anti-tumor immunity by the brain’s reward system. Nat. Commun. 9, 2723 (2018).
Mauffrey, P. et al. Progenitors from the central nervous system drive neurogenesis in cancer. Nature 569, 672–678 (2019).
Carloni, S. et al. Identification of a choroid plexus vascular barrier closing during intestinal inflammation. Science 374, 439–448 (2021).
Lutgendorf, S. K. et al. Social isolation is associated with elevated tumor norepinephrine in ovarian carcinoma patients. Brain Behav. Immun. 25, 250–255 (2011).
Campbell, J. P. et al. Stimulation of host bone marrow stromal cells by sympathetic nerves promotes breast cancer bone metastasis in mice. PLoS Biol. 10, e1001363 (2012).
Sloan, E. K. et al. The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer Res. 70, 7042–7052 (2010).
Chang, A. et al. β2-Adrenoceptors on tumor cells play a critical role in stress-enhanced metastasis in a mouse model of breast cancer. Brain Behav. Immun. 57, 106–115 (2016).
Hassan, S. et al. Behavioral stress accelerates prostate cancer development in mice. J. Clin. Investig. 123, 874–886 (2013).
Madden, K. S., Szpunar, M. J. & Brown, E. B.β-Adrenergic receptors (β-AR) regulate VEGF and IL-6 production by divergent pathways in high β-AR-expressing breast cancer cell lines. Breast Cancer Res. Treat. 130, 747–758 (2011).
Eng, J. W. et al. Housing temperature-induced stress drives therapeutic resistance in murine tumour models through β2-adrenergic receptor activation. Nat. Commun. 6, 6426 (2015).
Kim-Fuchs, C. et al. Chronic stress accelerates pancreatic cancer growth and invasion: a critical role for beta-adrenergic signaling in the pancreatic microenvironment. Brain Behav. Immun. 40, 40–47 (2014).
Pasquier, E. et al. β-Blockers increase response to chemotherapy via direct antitumour and anti-angiogenic mechanisms in neuroblastoma. Br. J. Cancer 108, 2485–2494 (2013).
Wolter, J. K. et al. Anti-tumor activity of the beta-adrenergic receptor antagonist propranolol in neuroblastoma. Oncotarget 5, 161–172 (2014).
Hasegawa, H. & Saiki, I. Psychosocial stress augments tumor development through β-adrenergic activation in mice. Jpn. J. Cancer Res. 93, 729–735 (2002).
Goldfarb, Y. et al. Improving postoperative immune status and resistance to cancer metastasis: a combined perioperative approach of immunostimulation and prevention of excessive surgical stress responses. Ann. Surg. 253, 798–810 (2011).
Vanhecke, E. et al. Brain-derived neurotrophic factor and neurotrophin-4/5 are expressed in breast cancer and can be targeted to inhibit tumor cell survival. Clin. Cancer Res. 17, 1741–1752 (2011).
Dobrenis, K., Gauthier, L. R., Barroca, V. & Magnon, C. Granulocyte colony-stimulating factor off-target effect on nerve outgrowth promotes prostate cancer development. Int. J. Cancer 136, 982–988 (2015).
Jiang, C. C. et al. Tumor innervation is triggered by endoplasmic reticulum stress. Oncogene https://doi.org/10.1038/s41388-021-02108-6 (2021).
Isaacs, J. T. Cancer. Prostate cancer takes nerve. Science 341, 134–135 (2013).
Allen, J. K. et al. Sustained adrenergic signaling promotes intratumoral innervation through BDNF induction. Cancer Res. 78, 3233–3242 (2018).
Madeo, M. et al. Cancer exosomes induce tumor innervation. Nat. Commun. 9, 4284 (2018).
Deborde, S. et al. Schwann cells induce cancer cell dispersion and invasion. J. Clin. Investig. 126, 1538–1554 (2016).
Roger, E. et al. Schwann cells support oncogenic potential of pancreatic cancer cells through TGFβ signaling. Cell Death Dis. 10, 886 (2019).
Ferdoushi, A. et al. Schwann cell stimulation of pancreatic cancer cells: a proteomic analysis. Front. Oncol. 10, 1601 (2020).
Anastasaki, C. et al. Neuronal hyperexcitability drives central and peripheral nervous system tumor progression in models of neurofibromatosis-1. Nat. Commun. 13, 2785 (2022).
Patritti-Cram, J., Coover, R. A., Jankowski, M. P. & Ratner, N. Purinergic signaling in peripheral nervous system glial cells. Glia 69, 1837–1851 (2021).
Armaiz-Pena, G. N. et al. Src activation by β-adrenoreceptors is a key switch for tumour metastasis. Nat. Commun. 4, 1403 (2013).
Lin, X., Luo, K., Lv, Z. & Huang, J. Beta-adrenoceptor action on pancreatic cancer cell proliferation and tumor growth in mice. Hepatogastroenterology 59, 584–588 (2012).
Pon, C. K., Lane, J. R., Sloan, E. K. & Halls, M. L. The β2-adrenoceptor activates a positive cAMP-calcium feedforward loop to drive breast cancer cell invasion. FASEB J. 30, 1144–1154 (2016).
Hara, M. R. et al. A stress response pathway regulates DNA damage through β2-adrenoreceptors and β-arrestin-1. Nature 477, 349–353 (2011).
Liu, J. et al. The effect of chronic stress on anti-angiogenesis of sunitinib in colorectal cancer models. Psychoneuroendocrinology 52, 130–142 (2015).
Deng, G. H. et al. Exogenous norepinephrine attenuates the efficacy of sunitinib in a mouse cancer model. J. Exp. Clin. Cancer Res. 33, 21 (2014).
Zhang, D., Ma, Q., Shen, S. & Hu, H. Inhibition of pancreatic cancer cell proliferation by propranolol occurs through apoptosis induction. The study of β-adrenoceptor antagonist’s anticancer effect in pancreatic cancer cell. Pancreas 38, 94–100 (2009).
Zhou, C. et al. Propranolol induced G0/G1/S phase arrest and apoptosis in melanoma cells via AKT/MAPK pathway. Oncotarget 7, 68314–68327 (2016).
Armaiz-Pena, G. N. et al. Adrenergic regulation of monocyte chemotactic protein 1 leads to enhanced macrophage recruitment and ovarian carcinoma growth. Oncotarget 6, 4266–4273 (2015).
Mohammadpour, H. et al. β2 adrenergic receptor-mediated signaling regulates the immunosuppressive potential of myeloid-derived suppressor cells. J. Clin. Investig. 129, 5537–5552 (2019).
Bucsek, M. J. et al. β-Adrenergic signaling in mice housed at standard temperatures suppresses an effector phenotype in CD8+ T cells and undermines checkpoint inhibitor therapy. Cancer Res. 77, 5639–5651 (2017).
Jean Wrobel, L. et al. Propranolol induces a favourable shift of anti-tumor immunity in a murine spontaneous model of melanoma. Oncotarget 7, 77825–77837 (2016).
Zahalka, A. H. et al. Adrenergic nerves activate an angio-metabolic switch in prostate cancer. Science 358, 321–326 (2017).
Chakroborty, D., Sarkar, C., Basu, B., Dasgupta, P. S. & Basu, S. Catecholamines regulate tumor angiogenesis. Cancer Res. 69, 3727–3730 (2009).
Yang, E. V. et al. Norepinephrine up-regulates the expression of vascular endothelial growth factor, matrix metalloproteinase (MMP)-2, and MMP-9 in nasopharyngeal carcinoma tumor cells. Cancer Res. 66, 10357–10364 (2006).
Le, C. P. et al. Chronic stress in mice remodels lymph vasculature to promote tumour cell dissemination. Nat. Commun. 7, 10634 (2016).
Pasquier, E. et al. Propranolol potentiates the anti-angiogenic effects and anti-tumor efficacy of chemotherapy agents: implication in breast cancer treatment. Oncotarget 2, 797–809 (2011).
Raju, B., Haug, S. R., Ibrahim, S. O. & Heyeraas, K. J. Sympathectomy decreases size and invasiveness of tongue cancer in rats. Neuroscience 149, 715–725 (2007).
Nagaraja, A. S. et al. Adrenergic-mediated increases in INHBA drive CAF phenotype and collagens. JCI Insight https://doi.org/10.1172/jci.insight.93076 (2017).
Calvani, M. et al. Norepinephrine promotes tumor microenvironment reactivity through beta3-adrenoreceptors during melanoma progression. Oncotarget 6, 4615–4632 (2015).
Gyamfi, J., Eom, M., Koo, J. S. & Choi, J. Multifaceted roles of interleukin-6 in adipocyte-breast cancer cell interaction. Transl. Oncol. 11, 275–285 (2018).
Petruzzelli, M. et al. A switch from white to brown fat increases energy expenditure in cancer-associated cachexia. Cell Metab. 20, 433–447 (2014).
Cao, L. et al. Environmental and genetic activation of a brain-adipocyte BDNF/leptin axis causes cancer remission and inhibition. Cell 142, 52–64 (2010).
Shan, T. et al. Novel regulatory program for norepinephrine-induced epithelial-mesenchymal transition in gastric adenocarcinoma cell lines. Cancer Sci. 105, 847–856 (2014).
Lu, Y. J. et al. Isoprenaline induces epithelial-mesenchymal transition in gastric cancer cells. Mol. Cell. Biochem. 408, 1–13 (2015).
Pu, J. et al. Adrenaline promotes epithelial-to-mesenchymal transition via HuR-TGFβ regulatory axis in pancreatic cancer cells and the implication in cancer prognosis. Biochem. Biophys. Res. Commun. 493, 1273–1279 (2017).
Zhang, J. et al. Norepinephrine induced epithelial-mesenchymal transition in HT-29 and A549 cells in vitro. J. Cancer Res. Clin. Oncol. 142, 423–435 (2016).
Lemeshow, S. et al. β-Blockers and survival among Danish patients with malignant melanoma: a population-based cohort study. Cancer Epidemiol. Biomark. Prev. 20, 2273–2279 (2011).
De Giorgi, V. et al. Treatment with β-blockers and reduced disease progression in patients with thick melanoma. Arch. Intern. Med. 171, 779–781 (2011).
Kokolus, K. M. et al. Beta blocker use correlates with better overall survival in metastatic melanoma patients and improves the efficacy of immunotherapies in mice. Oncoimmunology 7, e1405205 (2018).
Udumyan, R. et al. Beta-blocker drug use and survival among patients with pancreatic adenocarcinoma. Cancer Res. 77, 3700–3707 (2017).
Grytli, H. H., Fagerland, M. W., Fossa, S. D. & Tasken, K. A. Association between use of β-blockers and prostate cancer-specific survival: a cohort study of 3561 prostate cancer patients with high-risk or metastatic disease. Eur. Urol. https://doi.org/10.1016/j.eururo.2013.01.007 (2013).
Grytli, H. H., Fagerland, M. W., Fossa, S. D., Tasken, K. A. & Haheim, L. L. Use of β-blockers is associated with prostate cancer-specific survival in prostate cancer patients on androgen deprivation therapy. Prostate 73, 250–260 (2013).
Melhem-Bertrandt, A. et al. Beta-blocker use is associated with improved relapse-free survival in patients with triple-negative breast cancer. J. Clin. Oncol. 29, 2645–2652 (2011).
Barron, T. I., Connolly, R. M., Sharp, L., Bennett, K. & Visvanathan, K. Beta blockers and breast cancer mortality: a population- based study. J. Clin. Oncol. 29, 2635–2644 (2011).
Botteri, E. et al. Therapeutic effect of beta-blockers in triple-negative breast cancer postmenopausal women. Breast Cancer Res. Treat. 140, 567–575 (2013).
Powe, D. G. & Entschladen, F. Targeted therapies: using β-blockers to inhibit breast cancer progression. Nat. Rev. Clin. Oncol. 8, 511–512 (2011).
Diaz, E. S., Karlan, B. Y. & Li, A. J. Impact of beta blockers on epithelial ovarian cancer survival. Gynecol. Oncol. 127, 375–378 (2012).
Jansen, L., Hoffmeister, M., Arndt, V., Chang-Claude, J. & Brenner, H. Stage-specific associations between beta blocker use and prognosis after colorectal cancer. Cancer 120, 1178–1186 (2014).
Wang, H. M. et al. Improved survival outcomes with the incidental use of beta-blockers among patients with non-small-cell lung cancer treated with definitive radiation therapy. Ann. Oncol. 24, 1312–1319 (2013).
Shi, D. D. et al. Therapeutic avenues for cancer neuroscience: translational frontiers and clinical opportunities. Lancet Oncol. 23, e62–e74 (2022).
Hiller, J. G. et al. Preoperative β-blockade with propranolol reduces biomarkers of metastasis in breast cancer: a phase II randomized trial. Clin. Cancer Res. 26, 1803–1811 (2020).
Shaashua, L. et al. Perioperative COX-2 and β-adrenergic blockade improves metastatic biomarkers in breast cancer patients in a phase-II randomized trial. Clin. Cancer Res. 23, 4651–4661 (2017).
De Giorgi, V. et al. Propranolol for off-label treatment of patients with melanoma: results from a cohort study. JAMA Oncol. 4, e172908 (2018).
Knight, J. M. et al. Propranolol inhibits molecular risk markers in HCT recipients: a phase 2 randomized controlled biomarker trial. Blood Adv. 4, 467–476 (2020).
Renz, B. W. et al. Cholinergic signaling via muscarinic receptors directly and indirectly suppresses pancreatic tumorigenesis and cancer stemness. Cancer Discov. 8, 1458–1473 (2018).
Koppelmans, V. et al. Neuropsychological performance in survivors of breast cancer more than 20 years after adjuvant chemotherapy. J. Clin. Oncol. 30, 1080–1086 (2012).
Qi, Y. et al. Adiponectin acts in the brain to decrease body weight. Nat. Med. 10, 524–529 (2004).
Nakazato, M. et al. A role for ghrelin in the central regulation of feeding. Nature 409, 194–198 (2001).
Myers, M. G. Jr., Munzberg, H., Leinninger, G. M. & Leshan, R. L. The geometry of leptin action in the brain: more complicated than a simple ARC. Cell Metab. 9, 117–123 (2009).
Myers, M. G. Jr, Leibel, R. L., Seeley, R. J. & Schwartz, M. W. Obesity and leptin resistance: distinguishing cause from effect. Trends Endocrinol. Metab. 21, 643–651 (2010).
Lauby-Secretan, B. et al. Body fatness and cancer — viewpoint of the IARC Working Group. N. Engl. J. Med. 375, 794–798 (2016).
Garofalo, C. et al. Increased expression of leptin and the leptin receptor as a marker of breast cancer progression: possible role of obesity-related stimuli. Clin. Cancer Res. 12, 1447–1453 (2006).
Ringel, A. E. et al. Obesity shapes metabolism in the tumor microenvironment to suppress anti-tumor immunity. Cell 183, 1848–1866 (2020).
Schaffler, A., Scholmerich, J. & Buechler, C. Mechanisms of disease: adipokines and breast cancer — endocrine and paracrine mechanisms that connect adiposity and breast cancer. Nat. Clin. Pract. Endocrinol. Metab. 3, 345–354 (2007).
Nieman, K. M. et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat. Med. 17, 1498–1503 (2011).
Ishikawa, M., Kitayama, J. & Nagawa, H. Enhanced expression of leptin and leptin receptor (OB-R) in human breast cancer. Clin. Cancer Res. 10, 4325–4331 (2004).
Wang, T. et al. JAK/STAT3-regulated fatty acid β-oxidation is critical for breast cancer stem cell self-renewal and chemoresistance. Cell Metab. 27, 1357 (2018).
Leinninger, G. M. et al. Leptin acts via leptin receptor-expressing lateral hypothalamic neurons to modulate the mesolimbic dopamine system and suppress feeding. Cell Metab. 10, 89–98 (2009).
Xu, B. & Xie, X. Neurotrophic factor control of satiety and body weight. Nat. Rev. Neurosci. 17, 282–292 (2016).
Wang, P. et al. A leptin-BDNF pathway regulating sympathetic innervation of adipose tissue. Nature 583, 839–844 (2020).
Shimizu, Y. et al. Increased plasma ghrelin level in lung cancer cachexia. Clin. Cancer Res. 9, 774–778 (2003).
Wei, T., Ye, P., Peng, X., Wu, L. L. & Yu, G. Y. Circulating adiponectin levels in various malignancies: an updated meta-analysis of 107 studies. Oncotarget 7, 48671–48691 (2016).
Gahete, M. D. et al. A novel human ghrelin variant (In1-ghrelin) and ghrelin-O-acyltransferase are overexpressed in breast cancer: potential pathophysiological relevance. PLoS ONE 6, e23302 (2011).
Au, C. C., Furness, J. B. & Brown, K. A. Ghrelin and breast cancer: emerging roles in obesity, estrogen regulation, and cancer. Front. Oncol. 6, 265 (2016).
Olin, J. J. Cognitive function after systemic therapy for breast cancer. Oncology 15, 613–618 (2001).
Cheung, Y. T. et al. Association of proinflammatory cytokines and chemotherapy-associated cognitive impairment in breast cancer patients: a multi-centered, prospective, cohort study. Ann. Oncol. 26, 1446–1451 (2015).
Walker, A. K. et al. Low dose aspirin blocks breast cancer-induced cognitive impairment in mice. PLoS ONE 13, e0208593 (2018).
Sinha, S. et al. PanIN neuroendocrine cells promote tumorigenesis via neuronal cross-talk. Cancer Res. 77, 1868–1879 (2017).
Mantyh, P. W. Cancer pain and its impact on diagnosis, survival and quality of life. Nat. Rev. Neurosci. 7, 797–809 (2006).
Chen, P. et al. Olfactory sensory experience regulates gliomagenesis via neuronal IGF1. Nature 606, 550–556 (2022).
Schmidt, B. L. The neurobiology of cancer pain. Neuroscientist 20, 546–562 (2014).
Schmidt, B. L. What pain tells us about cancer. Pain 156, S32–S34 (2015).
Bortolin, A., Neto, E. & Lamghari, M. Calcium signalling in breast cancer associated bone pain. Int. J. Mol. Sci. https://doi.org/10.3390/ijms23031902 (2022).
Campos, C. A. et al. Cancer-induced anorexia and malaise are mediated by CGRP neurons in the parabrachial nucleus. Nat. Neurosci. 20, 934–942 (2017).
Palmiter, R. D. The parabrachial nucleus: CGRP neurons function as a general alarm. Trends Neurosci. 41, 280–293 (2018).
Jimenez-Andrade, J. M. et al. Pathological sprouting of adult nociceptors in chronic prostate cancer-induced bone pain. J. Neurosci. 30, 14649–14656 (2010).
Balood, M. et al. Nociceptor neurons affect cancer immunosurveillance. Nature 611, 405–412 (2022).
Sklar, L. S. & Anisman, H. Social stress influences tumor growth. Psychosom. Med. 42, 347–365 (1980).
Sephton, S. & Spiegel, D. Circadian disruption in cancer: a neuroendocrine-immune pathway from stress to disease. Brain Behav. Immun. 17, 321–328 (2003).
Engler, H., Bailey, M. T., Engler, A. & Sheridan, J. F. Effects of repeated social stress on leukocyte distribution in bone marrow, peripheral blood and spleen. J. Neuroimmunol. 148, 106–115 (2004).
Engler, H. et al. Effects of social stress on blood leukocyte distribution: the role of α- and β-adrenergic mechanisms. J. Neuroimmunol. 156, 153–162 (2004).
Soleyman-Jahi, S. et al. Attribution of ghrelin to cancer; attempts to unravel an apparent controversy. Front. Oncol. 9, 1014 (2019).
Engblom, C. et al. Osteoblasts remotely supply lung tumors with cancer-promoting SiglecF(high) neutrophils. Science https://doi.org/10.1126/science.aal5081 (2017).
Lakritz, J. R. et al. Gut bacteria require neutrophils to promote mammary tumorigenesis. Oncotarget 6, 9387–9396 (2015).
De Vadder, F. et al. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell 156, 84–96 (2014).
Kaelberer, M. M. et al. A gut-brain neural circuit for nutrient sensory transduction. Science https://doi.org/10.1126/science.aat5236 (2018).
Williams, E. K. et al. Sensory neurons that detect stretch and nutrients in the digestive system. Cell 166, 209–221 (2016).
Zhang, X. et al. Brain control of humoral immune responses amenable to behavioural modulation. Nature 581, 204–208 (2020).
de Kloet, A. D. & Herman, J. P. Fat-brain connections: adipocyte glucocorticoid control of stress and metabolism. Front. Neuroendocrinol. 48, 50–57 (2018).
Abu Rmaileh, A. et al. DPYSL2 interacts with JAK1 to mediate breast cancer cell migration. J. Cell Biol. https://doi.org/10.1083/jcb.202106078 (2022).
Binnewies, M. et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat. Med. 24, 541–550 (2018).
Turley, S. J., Cremasco, V. & Astarita, J. L. Immunological hallmarks of stromal cells in the tumour microenvironment. Nat. Rev. Immunol. 15, 669–682 (2015).
Cao, Y. & Langer, R. A review of Judah Folkman’s remarkable achievements in biomedicine. Proc. Natl Acad. Sci. USA 105, 13203–13205 (2008).
Folkman, J. Angiogenesis: an organizing principle for drug discovery? Nat. Rev. Drug Discov. 6, 273–286 (2007).
Paez-Ribes, M. et al. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell 15, 220–231 (2009).
Ebos, J. M. et al. Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell 15, 232–239 (2009).
Van der Veldt, A. A. et al. Rapid decrease in delivery of chemotherapy to tumors after anti-VEGF therapy: implications for scheduling of anti-angiogenic drugs. Cancer Cell 21, 82–91 (2012).
Schoenfeld, A. J. & Hellmann, M. D. Acquired resistance to immune checkpoint inhibitors. Cancer Cell 37, 443–455 (2020).
Van der Gucht, K. et al. Effects of a mindfulness-based intervention on cancer-related cognitive impairment: results of a randomized controlled functional magnetic resonance imaging pilot study. Cancer 126, 4246–4255 (2020).
Bower, J. E. et al. Mindfulness meditation for younger breast cancer survivors: a randomized controlled trial. Cancer 121, 1231–1240 (2015).
Wurtzen, H. et al. Mindfulness significantly reduces self-reported levels of anxiety and depression: results of a randomised controlled trial among 336 Danish women treated for stage I-III breast cancer. Eur. J. Cancer 49, 1365–1373 (2013).
Antoni, M. H. et al. The influence of bio-behavioural factors on tumour biology: pathways and mechanisms. Nat. Rev. Cancer 6, 240–248 (2006).
Bower, J. E. et al. Prometastatic molecular profiles in breast tumors from socially isolated women. JNCI Cancer Spectr. 2, pky029 (2018).
Cole, S. W. New challenges in psycho-oncology: neural regulation of the cancer genome. Psychooncology 27, 2305–2309 (2018).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT02944201 (2019).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT03861598 (2019).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT03384836 (2017).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT03838029 (2019).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT03919461 (2019).
Hiller, J. G. et al. Preoperative β-blockade with propranolol reduces biomarkers of metastasis in breast cancer: a phase II randomized trial. Clin. Cancer Res. 15, 1803–1811 (2020).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT03122444 (2017).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT00667121 (2008).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT03919292 (2019).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT04310176 (2020).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT01530373 (2012).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT02609828 (2015).
Somatilaka, B. N., Sadek, A., McKay, R. M. & Le, L. Q. Malignant peripheral nerve sheath tumor: models, biology, and translation. Oncogene 41, 2405–2421 (2022).
Patritti Cram, J. et al. P2RY14 cAMP signaling regulates Schwann cell precursor self-renewal, proliferation, and nerve tumor initiation in a mouse model of neurofibromatosis. eLife https://doi.org/10.7554/eLife.73511 (2022).
Rao, V. et al. Chemobrain: a review on mechanistic insight, targets and treatments. Adv. Cancer Res. 155, 29–76 (2022).
Gibson, E. M. & Monje, M. Emerging mechanistic underpinnings and therapeutic targets for chemotherapy-related cognitive impairment. Curr. Opin. Oncol. 31, 531–539 (2019).
Geraghty, A. C. et al. Loss of adaptive myelination contributes to methotrexate chemotherapy-related cognitive impairment. Neuron 103, 250–265 (2019).
Gibson, E. M. et al. Methotrexate chemotherapy induces persistent tri-glial dysregulation that underlies chemotherapy-related cognitive impairment. Cell 176, 43–55 (2019).
Acknowledgements
The authors thank P. Jobling, University of Newcastle, Australia, for critical reading of the manuscript.
Author information
Authors and Affiliations
Contributions
C.M. conceived and researched data for the article and wrote, reviewed and edited the manuscript before submission. H.H. wrote, reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Cancer thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Financial support
C.M. National Institute of Health and Medical Research (INSERM), National Institute of Cancer (INCA- PLBIO), Cancéropôle Ile-de-France, Foundation for cancer Research (ARC), University of Paris-Cité, University of Paris-Saclay, Atomic Energy Commission (CEA), Sanofi iAward Europe, France. H.H. National Health and Medical Research Council (NHMRC) and the Mark Hughes Foundation, Australia.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- β-Adrenergic pathway
-
Intracellular signalling activated by the stimulation of G protein-coupled, β-adrenergic receptors by epinephrine or norepinephrine.
- Adipokines
-
Cytokines secreted by adipose tissue that can function in a paracrine and endocrine manner.
- Adrenergic nerves
-
Nerves for which the neurotransmitter is either epinephrine, norepinephrine, or dopamine.
- Adrenergic splanchnic division
-
Paired autonomic nerves that carry both visceral sympathetic and sensory fibres.
- Afferent signals
-
Neuronal signals carried from the peripheral nervous system to the central nervous system.
- Amygdala
-
Brain area considered as the integrative centre for emotions, emotional behaviour and motivation.
- Angiogenesis inhibitors
-
Compounds that inhibit the growth of new blood vessels.
- Astrocytes
-
Star-shaped and supportive glial cells of the central nervous system.
- Autonomic nervous system
-
Part of the nervous system responsible for the control of bodily functions that are not consciously directed, such as breathing, heartbeat and digestion.
- Axonogenesis
-
Process by which neural extensions, known as axons, are generated. In cancer, tumours build up their own autonomic nerve network through a dynamic axonal outgrowth of pre-existing autonomic nerve fibres in the organ where the tumour initiates.
- Calcitonin gene-related peptide
-
CGRP. Peptide produced by sensory neurons in both the central and peripheral nervous systems that induces dilatation of blood vessels.
- Catecholamines
-
Neurotransmitters produced in the adrenal medulla and the postganglionic fibres of the sympathetic nervous system; the main catecholamines are epinephrine (also known as adrenaline), norepinephrine (also known as noradrenaline) and dopamine.
- Cholinergic nerve fibres
-
Nerve fibres that mainly use acetylcholine as a neurotransmitter.
- Efferent neural signals
-
Neuronal signals carried from the central nervous system to the peripheral nervous system.
- Episodic autobiographical memory retrieval
-
Remembering or re-experiencing a specific personal event from the past.
- Field potentials
-
Transient electrical signals generated in the nervous system.
- Glutamatergic neurotransmission
-
Transmission of information between neurons using glutamate as a neurotransmitter.
- Hippocampus
-
Central brain area that is essential for learning, emotions and memory.
- Hypothalamic–pituitary–adrenal (HPA) axis
-
A neuroendocrine system that mediates glucocorticoid release through molecular interactions among the hypothalamus (a region of the brain located below the thalamus), the pituitary gland (a pea-shaped structure located below the hypothalamus) and the adrenal glands (small conical organs on top of the kidneys).
- Immune-checkpoint inhibitors
-
Drugs used in immunotherapy of cancer to restore the function of the immune system.
- Immunotherapies
-
The treatment of disease by activating or suppressing the immune system.
- Lateral hypothalamus
-
LH. Brain area mainly involved in the regulation of feeding behaviour.
- Leptomeningeal microenvironment
-
Refers to leptomeninges, the two innermost layers of tissue that cover the brain and spinal cord.
- Locus coeruleus–noradrenergic system
-
Cluster of cells in the brainstem that is the main source of the neurotransmitter norepinephrine in the brain.
- Mesencephalic periaqueductal grey region
-
Interface between the forebrain and the lower brainstem that has a role in integrated behavioural responses to internal or external stressors such as pain or threat.
- Microglia
-
A specialized population of phagocytic cells, located in the central nervous system.
- Mindfulness-based therapy
-
A psychotherapeutic approach that uses meditative practices based on awareness of internal thoughts, feelings and emotions.
- Muscarinic cholinergic receptors
-
Membrane protein receptors involved in the transmission of nervous signals in the parasympathetic cholinergic nervous system.
- Myelination
-
Formation of a myelin sheath, which is made of proteins and lipids, around certain nerves, and allows nerve impulses to travel faster.
- Nerve fibres
-
Individual neural extensions also known as axons.
- Nerve sheath tumours
-
Tumours from the cells that form the sheath covering certain peripheral nerves.
- Neural cells
-
Differentiated cells of the nervous system, also called neurons.
- Neural projections
-
Processes extending from a neural cell, such as axons or dendrites, that are collectively called neurites.
- Neuroendocrine neurons
-
Neurons that can release neurohormones following neuronal stimulation.
- Neurogenic area
-
An area in the brain where neurogenesis, the process by which new neurons are formed, occurs.
- Neurotrophic growth factors
-
Peptides primarily involved in the regulation of survival, growth and differentiation of neurons.
- Neurotropism
-
Ability to invade or attract neural tissues.
- Nociception
-
Perception or sensation of pain.
- Noradrenergic neurons
-
Neurons that use norepinephrine (also known as noradrenaline) as a neurotransmitter.
- Nucleus of the solitary tract
-
Group of sensory neurons that are located in the dorsomedial medulla of the brain.
- Oligodendrocytes
-
Category of glial cells producing the myelin in the central nervous system.
- Orexinergic neurons
-
Neurons that release orexin, a peptide that regulates arousal, wakefulness and appetite.
- Parabrachial nucleus
-
Area located in the dorsolateral pons of the brain and working as a sensory relay that receives visceral, nociceptive and thermoreceptive inputs from the periphery and transfers the information to the hypothalamus and amygdala.
- Parasympathetic nerve fibres
-
Nerve fibres from the parasympathetic division of the autonomic nervous system, which is responsible for the rest and digestion response of the body.
- Peptidergic
-
Describing neurons that secrete peptides as their neurotransmitters.
- Phase shifts
-
Deregulation of circadian rhythms that originate in the suprachiasmatic nucleus of the brain, leading to a shift in the sleep or awake time.
- Prefrontal cortex
-
PFC. Region of the brain that makes up the frontal area of the frontal lobe and is mainly involved in mediating complex cognitive processes.
- Purinergic signalling
-
Extracellular signalling mediated by purine nucleotides and nucleosides such as adenosine or adenosine triphosphate (ATP).
- Retinohypothalamic tract
-
Light-initiated signalling pathway that signals from the retina to the suprachiasmatic nuclei of the hypothalamus in the brain.
- Schwann cells
-
Glial cells of the peripheral nervous system that help separate and insulate nerve cells.
- Seed and soil theory
-
A hypothesis that states that metastatic tumour cells can only grow at a site with a favourable local tissue microenvironment, just like a seed will only grow if it lands on fertile soil.
- Stereotactic activation
-
Electronically guided activation.
- Substantia nigra
-
Brain region that is part of the basal ganglia and is involved in the production of the neurotransmitter dopamine.
- Suprachiasmatic nucleus
-
SCN. Bilateral brain area located in the anterior part of the hypothalamus that is involved in the control of circadian rhythms.
- Sympathectomy
-
Surgical procedure during which at least one sympathetic nerve or sympathetic ganglion is removed.
- Sympathetic nervous system
-
Part of the autonomic nervous system that is best known for its role in responding to dangerous or stressful situations.
- Sympatho–adrenal system
-
SAS. Physiological connection between the sympathetic nervous system and the adrenal medulla that regulates the release of catecholamines in response to environmental stimuli.
- Thymic involution
-
Shrinking of the thymus that can occur naturally with age or acutely, as a consequence of stress, chemotherapy or other factors.
- Tumour neo-angiogenesis
-
Formation of new blood vessels in the tumour microenvironment.
- Viscero-sensory relays
-
Direct and indirect connections between sensory nerves of the autonomic nervous system and an organ.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Magnon, C., Hondermarck, H. The neural addiction of cancer. Nat Rev Cancer 23, 317–334 (2023). https://doi.org/10.1038/s41568-023-00556-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41568-023-00556-8
This article is cited by
-
Alpha5 nicotine acetylcholine receptor subunit promotes intrahepatic cholangiocarcinoma metastasis
Signal Transduction and Targeted Therapy (2024)
-
Eph receptors and ephrins in cancer progression
Nature Reviews Cancer (2024)