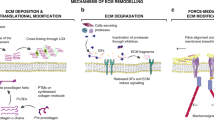
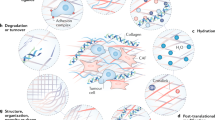
Abstract
Kallikrein-related peptidases (KLKs) are critical regulators of the tumour microenvironment. KLKs are proteolytic enzymes regulating multiple functions of bioactive molecules including hormones and growth factors, membrane receptors and the extracellular matrix architecture involved in cancer progression and metastasis. Perturbations of the proteolytic cascade generated by these peptidases, and their downstream signalling actions, underlie tumour emergence or blockade of tumour growth. Recent studies have also revealed their role in tumour immune suppression and resistance to cancer therapy. Here, we present an overview of the complex biology of the KLK family and its context-dependent nature in cancer, and discuss the different therapeutic strategies available to potentially target these proteases.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Kalinska, M., Meyer-Hoffert, U., Kantyka, T. & Potempa, J. Kallikreins — the melting pot of activity and function. Biochimie 122, 270–282 (2016).
Skala, W. et al. Structure–function analyses of human kallikrein-related peptidase 2 establish the 99-loop as master regulator of activity. J. Biol. Chem. 289, 34267–34283 (2014).
Guo, S. et al. A single glycan at the 99-loop of human kallikrein-related peptidase 2 regulates activation and enzymatic activity. J. Biol. Chem. 291, 593–604 (2016). This reference highlights the structural loops and the significance of glycosylation for KLK enzymatic function.
Koumandou, V. L. & Scorilas, A. Evolution of the plasma and tissue kallikreins, and their alternative splicing isoforms. PLoS ONE 8, e68074 (2013).
Filippou, P. S. et al. Expression profile of human tissue kallikrein 15 provides preliminary insights into its roles in the prostate and testis. Clin. Biochem. 59, 78–85 (2018).
Kishibe, M. Physiological and pathological roles of kallikrein-related peptidases in the epidermis. J. Dermatol. Sci. 95, 50–55 (2019).
Muytjens, C. M., Vasiliou, S. K., Oikonomopoulou, K., Prassas, I. & Diamandis, E. P. Putative functions of tissue kallikrein-related peptidases in vaginal fluid. Nat. Rev. Urol. 13, 596–607 (2016).
Stefanini, A. C., da Cunha, B. R., Henrique, T. & Tajara, E. H. Involvement of kallikrein-related peptidases in normal and pathologic processes. Dis. Markers 2015, 946572 (2015).
Di Paolo, C. T., Diamandis, E. P. & Prassas, I. The role of kallikreins in inflammatory skin disorders and their potential as therapeutic targets. Crit. Rev. Clin. Lab. Sci. 58, 1–16 (2020).
Filippou, P. S., Karagiannis, G. S., Musrap, N. & Diamandis, E. P. Kallikrein-related peptidases (KLKs) and the hallmarks of cancer. Crit. Rev. Clin. Lab. Sci. 53, 277–291 (2016).
Kryza, T., Silva, M. L., Loessner, D., Heuze-Vourc’h, N. & Clements, J. A. The kallikrein-related peptidase family: dysregulation and functions during cancer progression. Biochimie 122, 283–299 (2016).
Lawrence, M. G., Lai, J. & Clements, J. A. Kallikreins on steroids: structure, function, and hormonal regulation of prostate-specific antigen and the extended kallikrein locus. Endocr. Rev. 31, 407–446 (2010).
Avgeris, M., Mavridis, K. & Scorilas, A. Kallikrein-related peptidases in prostate, breast, and ovarian cancers: from pathobiology to clinical relevance. Biol. Chem. 393, 301–317 (2012).
Pasic, M. D., Olkhov, E., Bapat, B. & Yousef, G. M. Epigenetic regulation of kallikrein-related peptidases: there is a whole new world out there. Biol. Chem. 393, 319–330 (2012).
Adamopoulos, P. G., Kontos, C. K. & Scorilas, A. Discovery of novel transcripts of the human tissue kallikrein (KLK1) and kallikrein-related peptidase 2 (KLK2) in human cancer cells, exploiting next-generation sequencing technology. Genomics 111, 642–652 (2019).
Di Meo, A., Wang, C., Cheng, Y., Diamandis, E. P. & Yousef, G. M. The miRNA–kallikrein interaction: a mosaic of epigenetic regulation in cancer. Biol. Chem. 399, 973–982 (2018).
Masurier, N., Arama, D. P., El Amri, C. & Lisowski, V. Inhibitors of kallikrein-related peptidases: an overview. Med. Res. Rev. 38, 655–683 (2018).
Goettig, P. Effects of glycosylation on the enzymatic activity and mechanisms of proteases. Int. J. Mol. Sci. 17, 1969 (2016).
Guo, S., Briza, P., Magdolen, V., Brandstetter, H. & Goettig, P. Activation and activity of glycosylated KLKs 3, 4 and 11. Biol. Chem. 399, 1009–1022 (2018).
Srinivasan, S. et al. Prostate cancer risk-associated single-nucleotide polymorphism affects prostate-specific antigen glycosylation and its function. Clin. Chem. 65, e1–e9 (2019).
Gratacos-Mulleras, A. et al. Characterisation of the main PSA glycoforms in aggressive prostate cancer. Sci. Rep. 10, 18974 (2020).
Fortelny, N. et al. Network analyses reveal pervasive functional regulation between proteases in the human protease web. PLoS Biol. 12, e1001869 (2014).
Gong, W. et al. Characterization of kallikrein-related peptidase 4 (KLK4) mRNA expression in tumor tissue of advanced high-grade serous ovarian cancer patients. PLoS ONE 14, e0212968 (2019).
Tailor, P. D. et al. Diagnostic and prognostic biomarker potential of kallikrein family genes in different cancer types. Oncotarget 9, 17876–17888 (2018).
Peng, Q. et al. Biomarker implication of kallikrein-related peptidases as prognostic tissue substrates of poor survival in colorectal cancer. Cancer Cell Int. 20, 260 (2020).
Chang, J. S. et al. Kallikrein 5 overexpression is associated with poor prognosis in uterine cervical cancer. J. Gynecol. Oncol. 31, e78 (2020).
Gong, W. et al. Prognostic value of kallikrein-related peptidase 7 (KLK7) mRNA expression in advanced high-grade serous ovarian cancer. J. Ovarian Res. 13, 125 (2020).
Hua, Q. et al. Upregulation of KLK8 predicts poor prognosis in pancreatic cancer. Front Oncol 11, 624837 (2021).
Dorn, J. et al. Function and clinical relevance of kallikrein-related peptidases and other serine proteases in gynecological cancers. Crit. Rev. Clin. Lab. Sci. 51, 63–84 (2014).
Kolin, D. L. et al. Prognostic significance of human tissue kallikrein-related peptidases 11 and 15 in gastric cancer. Tumour Biol. 37, 437–446 (2016). Together with Gong et al. (2019), Tailor et al. (2018), Peng et al. (2020), Chang et al. (2020), Gong et al. (2020), Hua et al. (2021) and Dorn et al. (2014), this work highlights the significance of KLKs in tumour progression and metastasis and their clinical relevance.
Assel, M. J. et al. Kallikrein markers performance in pretreatment blood to predict early prostate cancer recurrence and metastasis after radical prostatectomy among very high-risk men. Prostate 80, 51–56 (2020).
Lunger, L. et al. KLK3 and TMPRSS2 for molecular lymph-node staging in prostate cancer patients undergoing radical prostatectomy. Prostate Cancer Prostatic Dis. 24, 362–369 (2020).
Schmitt, M. et al. Emerging clinical importance of the cancer biomarkers kallikrein-related peptidases (KLK) in female and male reproductive organ malignancies. Radiol. Oncol. 47, 319–329 (2013).
Halaoui, R. et al. Progressive polarity loss and luminal collapse disrupt tissue organization in carcinoma. Genes Dev. 31, 1573–1587 (2017).
Filippou, P. S. et al. Kallikrein-related peptidases protein expression in lymphoid tissues suggests potential implications in immune response. Clin. Biochem. 77, 41–47 (2020).
Prassas, I., Eissa, A., Poda, G. & Diamandis, E. P. Unleashing the therapeutic potential of human kallikrein-related serine proteases. Nat. Rev. Drug Discov. 14, 183–202 (2015).
Silva, L. M. et al. Integration of two in-depth quantitative proteomics approaches determines the kallikrein-related peptidase 7 (KLK7) degradome in ovarian cancer cell secretome. Mol. Cell Proteom. 18, 818–836 (2019).
Kapadia, C., Ghosh, M. C., Grass, L. & Diamandis, E. P. Human kallikrein 13 involvement in extracellular matrix degradation. Biochem. Biophys. Res. Commun. 323, 1084–1090 (2004).
Ghosh, M. C., Grass, L., Soosaipillai, A., Sotiropoulou, G. & Diamandis, E. P. Human kallikrein 6 degrades extracellular matrix proteins and may enhance the metastatic potential of tumour cells. Tumour Biol. 25, 193–199 (2004).
Michael, I. P. et al. Biochemical and enzymatic characterization of human kallikrein 5 (hK5), a novel serine protease potentially involved in cancer progression. J. Biol. Chem. 280, 14628–14635 (2005).
Dong, Y. et al. Metastasis of ovarian cancer is mediated by kallikrein related peptidases. Clin. Exp. Metastasis 31, 135–147 (2014).
Dong, Y. et al. Activation of membrane-bound proteins and receptor systems: a link between tissue kallikrein and the KLK-related peptidases. Biol. Chem. 395, 977–990 (2014).
Blaber, M., Yoon, H., Juliano, M. A., Scarisbrick, I. A. & Blaber, S. I. Functional intersection of the kallikrein-related peptidases (KLKs) and thrombostasis axis. Biol. Chem. 391, 311–320 (2010).
Beaufort, N. et al. Interplay of human tissue kallikrein 4 (hK4) with the plasminogen activation system: hK4 regulates the structure and functions of the urokinase-type plasminogen activator receptor (uPAR). Biol. Chem. 387, 217–222 (2006).
Sotiropoulou, G., Pampalakis, G. & Diamandis, E. P. Functional roles of human kallikrein-related peptidases. J. Biol. Chem. 284, 32989–32994 (2009).
Ohler, A., Debela, M., Wagner, S., Magdolen, V. & Becker-Pauly, C. Analyzing the protease web in skin: meprin metalloproteases are activated specifically by KLK4, 5 and 8 vice versa leading to processing of proKLK7 thereby triggering its activation. Biol. Chem. 391, 455–460 (2010).
Yoon, H. et al. Activation profiles and regulatory cascades of the human kallikrein-related peptidases. J. Biol. Chem. 282, 31852–31864 (2007).
Neuhaus, J. et al. Protease expression levels in prostate cancer tissue can explain prostate cancer-associated seminal biomarkers — an explorative concept study. Int. J. Mol. Sci. 18, 976 (2017).
Yoon, H., Blaber, S. I., Li, W., Scarisbrick, I. A. & Blaber, M. Activation profiles of human kallikrein-related peptidases by matrix metalloproteinases. Biol. Chem. 394, 137–147 (2013).
Loessner, D. et al. Combined expression of KLK4, KLK5, KLK6, and KLK7 by ovarian cancer cells leads to decreased adhesion and paclitaxel-induced chemoresistance. Gynecol. Oncol. 127, 569–578 (2012).
Dong, Y. et al. Kallikrein-related peptidase 7 promotes multicellular aggregation via the α5β1 integrin pathway and paclitaxel chemoresistance in serous epithelial ovarian carcinoma. Cancer Res. 70, 2624–2633 (2010).
Johnson, S. K., Ramani, V. C., Hennings, L. & Haun, R. S. Kallikrein 7 enhances pancreatic cancer cell invasion by shedding E-cadherin. Cancer 109, 1811–1820 (2007).
Ramani, V. C., Hennings, L. & Haun, R. S. Desmoglein 2 is a substrate of kallikrein 7 in pancreatic cancer. BMC Cancer 8, 373 (2008).
Jiang, R., Shi, Z., Johnson, J. J., Liu, Y. & Stack, M. S. Kallikrein-5 promotes cleavage of desmoglein-1 and loss of cell–cell cohesion in oral squamous cell carcinoma. J. Biol. Chem. 286, 9127–9135 (2011).
Schrader, C. H. et al. Kallikrein-related peptidase 6 regulates epithelial-to-mesenchymal transition and serves as prognostic biomarker for head and neck squamous cell carcinoma patients. Mol. Cancer 14, 107 (2015).
Delaunay, T. et al. Aberrant expression of kallikrein-related peptidase 7 is correlated with human melanoma aggressiveness by stimulating cell migration and invasion. Mol. Oncol. 11, 1330–1347 (2017).
Klucky, B. et al. Kallikrein 6 induces E-cadherin shedding and promotes cell proliferation, migration, and invasion. Cancer Res. 67, 8198–8206 (2007).
Veveris-Lowe, T. L. et al. Kallikrein 4 (hK4) and prostate-specific antigen (PSA) are associated with the loss of E-cadherin and an epithelial–mesenchymal transition (EMT)-like effect in prostate cancer cells. Endocr. Relat. Cancer 12, 631–643 (2005).
Kourtidis, A., Lu, R., Pence, L. J. & Anastasiadis, P. Z. A central role for cadherin signaling in cancer. Exp. Cell Res. 358, 78–85 (2017).
Reid, J. C. et al. Pericellular regulation of prostate cancer expressed kallikrein-related peptidases and matrix metalloproteinases by cell surface serine proteases. Am. J. Cancer Res. 7, 2257–2274 (2017).
Sahai, E. et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat. Rev. Cancer 20, 174–186 (2020).
Kryza, T. et al. Kallikrein-related peptidase 4 induces cancer-associated fibroblast features in prostate-derived stromal cells. Mol. Oncol. 11, 1307–1329 (2017).
Oikonomopoulou, K. et al. Kallikrein-mediated cell signalling: targeting proteinase-activated receptors (PARs). Biol. Chem. 387, 817–824 (2006).
Wojtukiewicz, M. Z., Hempel, D., Sierko, E., Tucker, S. C. & Honn, K. V. Protease-activated receptors (PARs) — biology and role in cancer invasion and metastasis. Cancer Metastasis Rev. 34, 775–796 (2015).
Hollenberg, M. D. KLKs and their hormone-like signaling actions: a new life for the PSA–KLK family. Biol. Chem. 395, 915–929 (2014).
Heuberger, D. M. & Schuepbach, R. A. Protease-activated receptors (PARs): mechanisms of action and potential therapeutic modulators in PAR-driven inflammatory diseases. Thromb. J. 17, 4 (2019).
Sebert, M., Sola-Tapias, N., Mas, E., Barreau, F. & Ferrand, A. Protease-activated receptors in the intestine: focus on inflammation and cancer. Front. Endocrinol. 10, 717 (2019).
Michel, N. et al. Growth and survival of lung cancer cells: regulation by kallikrein-related peptidase 6 via activation of proteinase-activated receptor 2 and the epidermal growth factor receptor. Biol. Chem. 395, 1015–1025 (2014).
Wang, W., Mize, G. J., Zhang, X. & Takayama, T. K. Kallikrein-related peptidase-4 initiates tumor–stroma interactions in prostate cancer through protease-activated receptor-1. Int. J. Cancer 126, 599–610 (2010).
Zhang, X. & Hwang, Y. S. Cancer-associated fibroblast stimulates cancer cell invasion in an interleukin-1 receptor (IL-1R)-dependent manner. Oncol. Lett. 18, 4645–4650 (2019).
Fuhrman-Luck, R. A., Loessner, D. & Clements, J. A. Kallikrein-related peptidases in prostate cancer: from molecular function to clinical application. EJIFCC 25, 269–281 (2014).
Dallas, S. L. et al. Preferential production of latent transforming growth factor β2 by primary prostatic epithelial cells and its activation by prostate-specific antigen. J. Cell Physiol. 202, 361–370 (2005).
Shahinian, H. et al. Secretome and degradome profiling shows that Kallikrein-related peptidases 4, 5, 6, and 7 induce TGFβ-1 signaling in ovarian cancer cells. Mol. Oncol. 8, 68–82 (2014). This work is the first cell-contextual proteomic and degradomic analysis for KLK4–KLK7 highlighting the significance of such studies in annotating the role of proteases in tumour biology.
Bellomo, C., Caja, L. & Moustakas, A. Transforming growth factor β as regulator of cancer stemness and metastasis. Br. J. Cancer 115, 761–769 (2016).
Costanza, B., Umelo, I. A., Bellier, J., Castronovo, V. & Turtoi, A. Stromal modulators of TGF-β in cancer. J. Clin. Med. 6, 7 (2017).
Ping, Q. R. et al. Cancer-associated fibroblasts: overview, progress, challenges, and directions. Cancer Gene Ther. 28, 984–999 (2021).
Cohen, P. et al. Prostate-specific antigen (PSA) is an insulin-like growth factor binding protein-3 protease found in seminal plasma. J. Clin. Endocrinol. Metab. 75, 1046–1053 (1992).
Matsumura, M. et al. Substrates of the prostate-specific serine protease prostase/KLK4 defined by positional-scanning peptide libraries. Prostate 62, 1–13 (2005).
Sutkowski, D. M. et al. Growth regulation of prostatic stromal cells by prostate-specific antigen. J. Natl Cancer Inst. 91, 1663–1669 (1999).
Mukai, S. et al. Activation of hepatocyte growth factor activator zymogen (pro-HGFA) by human kallikrein 1-related peptidases. FEBS J. 275, 1003–1017 (2008).
Reid, J. C. et al. In vitro evidence that KLK14 regulates the components of the HGF/Met axis, pro-HGF and HGF-activator inhibitor 1A and 1B. Biol. Chem. 397, 1299–1305 (2016).
Koh, S. A. & Lee, K. H. Function of hepatocyte growth factor in gastric cancer proliferation and invasion. Yeungnam Univ. J. Med. 37, 73–78 (2020).
Wilkinson, R. et al. Human kallikrein 4 signal peptide induces cytotoxic T cell responses in healthy donors and prostate cancer patients. Cancer Immunol. Immunother. 61, 169–179 (2012).
Japp, A. S. et al. Dysfunction of PSA-specific CD8+ T cells in prostate cancer patients correlates with CD38 and Tim-3 expression. Cancer Immunol. Immunother. 64, 1487–1494 (2015).
Karan, D. Formulation of the bivalent prostate cancer vaccine with surgifoam elicits antigen-specific effector T cells in PSA-transgenic mice. Vaccine 35, 5794–5798 (2017).
Schuster, H. et al. The immunopeptidomic landscape of ovarian carcinomas. Proc. Natl Acad. Sci. USA 114, E9942–E9951 (2017).
Ehrenfeld, P., Figueroa, C. D. & Bhoola, K. D. Kinins: kallikreins and kinins in cancer. Kinins 382, 77–89 (2012).
Weidmann, H. et al. The plasma contact system, a protease cascade at the nexus of inflammation, coagulation and immunity. Biochim. Biophys. Acta Mol. Cell. Res. 1864, 2118–2127 (2017).
Molina, L. et al. Stimulation of the bradykinin B1 receptor induces the proliferation of estrogen-sensitive breast cancer cells and activates the ERK1/2 signaling pathway. Breast Cancer Res. Treat. 118, 499–510 (2009).
Ehrenfeld, P. et al. Bioregulation of kallikrein-related peptidases 6, 10 and 11 by the kinin B1 receptor in breast cancer cells. Anticancer. Res. 34, 6925–6938 (2014).
Kodaira, H. et al. ANXA10 induction by interaction with tumor-associated macrophages promotes the growth of esophageal squamous cell carcinoma. Pathol. Int. 69, 135–147 (2019).
Grunberg, M. et al. Kallikrein-related peptidases are activators of the CC chemokine CCL14. Eur. J. Immunol. 48, 1592–1594 (2018).
Yamamoto, Y. et al. Tumour and immune cell dynamics explain the PSA bounce after prostate cancer brachytherapy. Br. J. Cancer 115, 195–202 (2016).
Manning, M. L., Williams, S. A., Jelinek, C. A., Kostova, M. B. & Denmeade, S. R. Proteolysis of complement factors iC3b and C5 by the serine protease prostate-specific antigen in prostatic fluid and seminal plasma. J. Immunol. 190, 2567–2574 (2013).
Roumenina, L. T., Daugan, M. V., Petitprez, F., Sautes-Fridman, C. & Fridman, W. H. Context-dependent roles of complement in cancer. Nat. Rev. Cancer 19, 698–715 (2019).
Oikonomopoulou, K. et al. Induction of complement C3a receptor responses by kallikrein-related peptidase 14. J. Immunol. 191, 3858–3866 (2013).
Cheteh, E. H. et al. Interleukin-6 derived from cancer-associated fibroblasts attenuates the p53 response to doxorubicin in prostate cancer cells. Cell Death Discov. 6, 42 (2020).
Johnson, J. J. et al. Protease-activated receptor-2 (PAR-2)-mediated NF-κB activation suppresses inflammation-associated tumor suppressor microRNAs in oral squamous cell carcinoma. J. Biol. Chem. 291, 6936–6945 (2016).
Scarisbrick, I. A. et al. Functional role of kallikrein 6 in regulating immune cell survival. PLoS ONE 6, e18376 (2011). This study points out the novel molecular mechanism involving kallikreins in lymphocyte survival and maintenance of cellular homeostasis.
Sotiropoulou, G. & Pampalakis, G. Kallikrein-related peptidases: bridges between immune functions and extracellular matrix degradation. Biol. Chem. 391, 321–331 (2010).
Chen, J.-Q., Liang, B.-H., Li, H.-P., Mo, Z.-Y. & Zhu, H.-L. Roles of kallikrein-related peptidase in epidermal barrier function and related skin diseases. J. Dermatol. Sci. 2, 150–155 (2019).
Chen, J. et al. Functional implications of cathelicidin antimicrobial protein in breast cancer and tumor-associated macrophage microenvironment. Biomolecules 10, 688 (2020).
Piktel, E. et al. The role of cathelicidin LL-37 in cancer development. Arch. Immunol. Ther. Exp. 64, 33–46 (2016).
Deraison, C. et al. LEKTI fragments specifically inhibit KLK5, KLK7, and KLK14 and control desquamation through a pH-dependent interaction. Mol. Biol. Cell 18, 3607–3619 (2007).
Briot, A. et al. Kallikrein 5 induces atopic dermatitis-like lesions through PAR2-mediated thymic stromal lymphopoietin expression in Netherton syndrome. J. Exp. Med. 206, 1135–1147 (2009).
Pampalakis, G. et al. The KLK5 protease suppresses breast cancer by repressing the mevalonate pathway. Oncotarget 5, 2390–2403 (2014).
Lim, S. C., Kee, K. H., Lee, M. J., Hong, R. & Han, S. I. Extracellular acidity-induced expression of Kallikrein-related peptidases 7 and 8 is involved in increased invasiveness of gastric cancer cells. Oncol. Rep. 43, 1705–1713 (2020). This study demonstrates that tumour invasion is, in part, regulated through the COX/KLK7 and KLK8 axis in gastric cancer.
Tian, J. et al. Identification of MFGE8 and KLK5/7 as mediators of breast tumorigenesis and resistance to COX-2 inhibition. Breast Cancer Res. 23, 23 (2021).
Ehrenfeld, P., Bhoola, K. D., Matus, C. E. & Figueroa, C. D. Functional interrelationships between the kallikrein-related peptidases family and the classical kinin system in the human neutrophil. Biol. Chem. 399, 925–935 (2018). This study highlights the importance of the bioregulation of KLKs by kinins.
Lizama, A. J. et al. Expression and bioregulation of the kallikrein-related peptidases family in the human neutrophil. Innate Immun. 21, 575–586 (2015).
Petrova, E. & Hovnanian, A. Advances in understanding of Netherton syndrome and therapeutic implications. Expert Opin. Orphan Drugs 8, 455–487 (2020).
Kiss, M. et al. IL1β promotes immune suppression in the tumor microenvironment independent of the inflammasome and gasdermin D. Cancer Immunol. Res. 9, 309–323 (2021).
Bhoola, K. et al. Kallikrein and kinin receptor expression in inflammation and cancer. Biol. Chem. 382, 77–89 (2001).
Chen, S. et al. Single-cell analysis reveals transcriptomic remodellings in distinct cell types that contribute to human prostate cancer progression. Nat. Cell Biol. 23, 87–98 (2021). This transcriptome profiling study in prostate primary tumours shows the involvement of KLK3 in micrometastasis.
Nauroy, P. & Nystrom, A. Kallikreins: essential epidermal messengers for regulation of the skin microenvironment during homeostasis, repair and disease. Matrix Biol. Plus 6–7, 100019 (2020).
Yoon, H. & Scarisbrick, I. A. Kallikrein-related peptidase 6 exacerbates disease in an autoimmune model of multiple sclerosis. Biol. Chem. 397, 1277–1286 (2016).
Kryza, T. et al. Human kallikrein-related peptidase 12 stimulates endothelial cell migration by remodeling the fibronectin matrix. Sci. Rep. 8, 6331 (2018).
Chadha, K. C. et al. Anti-angiogenic activity of PSA-derived peptides. Prostate 75, 1285–1299 (2015).
Lee, P., Chandel, N. S. & Simon, M. C. Cellular adaptation to hypoxia through hypoxia inducible factors and beyond. Nat. Rev. Mol. Cell Biol. 21, 268–283 (2020).
Horii, K. et al. Androgen-dependent gene expression of prostate-specific antigen is enhanced synergistically by hypoxia in human prostate cancer cells. Mol. Cancer Res. 5, 383–391 (2007).
Kryza, T. et al. Angiogenesis stimulated by human kallikrein-related peptidase 12 acting via a platelet-derived growth factor B-dependent paracrine pathway. FASEB J. 28, 740–751 (2014).
Yao, Y. et al. Tissue kallikrein-modified human endothelial progenitor cell implantation improves cardiac function via enhanced activation of akt and increased angiogenesis. Lab. Invest. 93, 577–591 (2013).
Giusti, B. et al. The antiangiogenic tissue kallikrein pattern of endothelial cells in systemic sclerosis. Arthritis Rheum. 52, 3618–3628 (2005).
Kryza, T. et al. Pro-angiogenic effect of human kallikrein-related peptidase 12 (KLK12) in lung endothelial cells does not depend on kinin-mediated activation of B2 receptor. Biol. Chem. 394, 385–391 (2012).
Groppa, E. et al. EphrinB2/EphB4 signaling regulates non-sprouting angiogenesis by VEGF. EMBO Rep. 19, e45054 (2018).
Lisle, J. E. et al. Murine, but not human, ephrin-B2 can be efficiently cleaved by the serine protease kallikrein-4: implications for xenograft models of human prostate cancer. Exp. Cell Res. 333, 136–146 (2015). Together with Silva et al. (2019), Kapadia et al. (2004), Ghosh et al. (2004), Michael et al. (2005), Beaufort et al. (2006) and Reid et al. (2017), this work reports the association of KLKs with cell surface proteins in promoting cancer.
Heidtmann, H. H. et al. Generation of angiostatin-like fragments from plasminogen by prostate-specific antigen. Br. J. Cancer 81, 1269–1273 (1999).
Koistinen, H. et al. Novel small molecule inhibitors for prostate-specific antigen. Prostate 68, 1143–1151 (2008).
Mattsson, J. M. et al. Proteolytic activity of prostate-specific antigen (PSA) towards protein substrates and effect of peptides stimulating PSA activity. PLoS ONE 9, e107819 (2014).
Fortier, A. H., Nelson, B. J., Grella, D. K. & Holaday, J. W. Antiangiogenic activity of prostate-specific antigen. J. Natl Cancer Inst. 91, 1635–1640 (1999).
Fortier, A. H. et al. Recombinant prostate specific antigen inhibits angiogenesis in vitro and in vivo. Prostate 56, 212–219 (2003). Together with Kryza et al. (2014) and Heidtmann et al. (1999), this work shows the effect of KLKs on angiogenesis.
Naidoo, S. & Raidoo, D. M. Angiogenesis in cervical cancer is mediated by HeLa metabolites through endothelial cell tissue kallikrein. Oncol. Rep. 22, 285–293 (2009).
Fuhrman-Luck, R. A., Stansfield, S. H., Stephens, C. R., Loessner, D. & Clements, J. A. Prostate cancer-associated kallikrein-related peptidase 4 activates matrix metalloproteinase-1 and thrombospondin-1. J. Proteome Res. 15, 2466–2478 (2016).
Jha, S. K. et al. KLK3/PSA and cathepsin D activate VEGF-C and VEGF-D. eLife 8, e44478 (2019). This study is the first to show the cleaving ability of KLKs on VEGF-C and VEGF-D, linking this molecular event to lymphangiogenesis.
Guillon-Munos, A. et al. Kallikrein-related peptidase 12 hydrolyzes matricellular proteins of the CCN family and modifies interactions of CCN1 and CCN5 with growth factors. J. Biol. Chem. 286, 25505–25518 (2011).
Jia, Q., Xu, B., Zhang, Y., Ali, A. & Liao, X. CCN family proteins in cancer: insight into their structures and coordination role in tumor microenvironment. Front. Genet. 12, 649387 (2021).
Caliendo, G. et al. Kallikrein protease activated receptor (PAR) axis: an attractive target for drug development. J. Med. Chem. 55, 6669–6686 (2012).
Filippou, P. S. et al. Biochemical characterization of human tissue kallikrein 15 and examination of its potential role in cancer. Clin. Biochem. 58, 108–115 (2018).
LaValley, D. J. et al. Matrix stiffness enhances VEGFR-2 internalization, signaling, and proliferation in endothelial cells. Converg. Sci. Phys. Oncol. 3, 044001 (2017).
Brassard-Jollive, N., Monnot, C., Muller, L. & Germain, S. In vitro 3D systems to model tumor angiogenesis and interactions with stromal cells. Front. Cell Dev. Biol. 8, 594903 (2020).
Winkler, J., Abisoye-Ogunniyan, A., Metcalf, K. J. & Werb, Z. Concepts of extracellular matrix remodelling in tumour progression and metastasis. Nat. Commun. 11, 5120 (2020).
Chou, R. H., Lin, S. C., Wen, H. C., Wu, C. W. & Chang, W. S. Epigenetic activation of human kallikrein 13 enhances malignancy of lung adenocarcinoma by promoting N-cadherin expression and laminin degradation. Biochem. Biophys. Res. Commun. 409, 442–447 (2011).
Ishige, S. et al. Decreased expression of kallikrein-related peptidase 13: possible contribution to metastasis of human oral cancer. Mol. Carcinog. 53, 557–565 (2014).
Zhu, S., Shi, J., Zhang, S. & Li, Z. KLK6 promotes growth, migration, and invasion of gastric cancer cells. J. Gastric Cancer 18, 356–367 (2018).
Chen, H. et al. Kallikrein 6 protease advances colon tumorigenesis via induction of the high mobility group A2 protein. Oncotarget 10, 6062–6078 (2019).
Pampalakis, G. et al. Biochemical pathways mediated by KLK6 protease in breast cancer. Mol. Oncol. 13, 2329–2343 (2019).
Pampalakis, G. et al. A tumor-protective role for human kallikrein-related peptidase 6 in breast cancer mediated by inhibition of epithelial-to-mesenchymal transition. Cancer Res. 69, 3779–3787 (2009).
Lei, S., Zhang, Q., Yin, F., He, X. & Wang, J. Expression and clinical significance of KLK5–8 in endometrial cancer. Am. J. Transl. Res. 11, 4180–4191 (2019).
Chen, E. et al. Analysis of expression and prognosis of KLK7 in ovarian cancer. Open Med. 15, 932–939 (2020).
Hua, Q. et al. KLK8 promotes the proliferation and metastasis of colorectal cancer via the activation of EMT associated with PAR1. Cell Death Dis. 12, 860 (2021).
Kaneko, N. et al. Differential roles of kallikrein-related peptidase 6 in malignant transformation and ΔNp63β-mediated epithelial–mesenchymal transition of oral squamous cell carcinoma. Oral. Oncol. 75, 148–157 (2017).
Gao, L. et al. Tissue kallikrein promotes prostate cancer cell migration and invasion via a protease-activated receptor-1-dependent signaling pathway. Biol. Chem. 391, 803–812 (2010).
Ramsay, A. J. et al. Prostatic trypsin-like kallikrein-related peptidases (KLKs) and other prostate-expressed tryptic proteinases as regulators of signalling via proteinase-activated receptors (PARs). Biol. Chem. 389, 653–668 (2008).
Ramsay, A. J. et al. Kallikrein-related peptidase 4 (KLK4) initiates intracellular signaling via protease-activated receptors (PARs). KLK4 and PAR-2 are co-expressed during prostate cancer progression. J. Biol. Chem. 283, 12293–12304 (2008).
Avgeris, M. & Scorilas, A. Kallikrein-related peptidases (KLKs) as emerging therapeutic targets: focus on prostate cancer and skin pathologies. Expert Opin. Ther. Targets 20, 801–818 (2016).
Takayama, T. K., McMullen, B. A., Nelson, P. S., Matsumura, M. & Fujikawa, K. Characterization of hK4 (prostase), a prostate-specific serine protease: activation of the precursor of prostate specific antigen (pro-PSA) and single-chain urokinase-type plasminogen activator and degradation of prostatic acid phosphatase. Biochemistry 40, 15341–15348 (2001).
Dong, Y. et al. Paclitaxel resistance and multicellular spheroid formation are induced by kallikrein-related peptidase 4 in serous ovarian cancer cells in an ascites mimicking microenvironment. PLoS ONE 8, e57056 (2013).
Loessner, D. et al. A bioengineered 3D ovarian cancer model for the assessment of peptidase-mediated enhancement of spheroid growth and intraperitoneal spread. Biomaterials 34, 7389–7400 (2013).
Tse, B. W. et al. KLK4 induces anti-tumor effects in human xenograft mouse models of orthotopic and metastatic prostate cancer. Cancers (Basel) 12, 3501 (2020).
Emami, N. & Diamandis, E. P. Potential role of multiple members of the kallikrein-related peptidase family of serine proteases in activating latent TGFβ1 in semen. Biol. Chem. 391, 85–95 (2010).
Roblek, M. et al. CCL2 is a vascular permeability factor inducing CCR2-dependent endothelial retraction during lung metastasis. Mol. Cancer Res. 17, 783–793 (2019).
Magnen, M. et al. Tissue kallikrein regulates alveolar macrophage apoptosis early in influenza virus infection. Am. J. Physiol. Lung Cell Mol. Physiol. 316, L1127–L1140 (2019).
Gao, J. et al. Kallikrein 4 is a potential mediator of cellular interactions between cancer cells and osteoblasts in metastatic prostate cancer. Prostate 67, 348–360 (2007).
Yang, F. et al. Stromal TGF-β signaling induces AR activation in prostate cancer. Oncotarget 5, 10854–10869 (2014).
Ghahremanifard, P., Chanda, A., Bonni, S. & Bose, P. TGF-β mediated immune evasion in cancer-spotlight on cancer-associated fibroblasts. Cancers (Basel) 12, 3650 (2020).
Ao, M. et al. Cross-talk between paracrine-acting cytokine and chemokine pathways promotes malignancy in benign human prostatic epithelium. Cancer Res. 67, 4244–4253 (2007).
Xie, F., Ling, L., van Dam, H., Zhou, F. & Zhang, L. TGF-β signaling in cancer metastasis. Acta Biochim. Biophys. Sin. 50, 121–132 (2018).
Nadiminty, N. et al. Prostate-specific antigen modulates genes involved in bone remodeling and induces osteoblast differentiation of human osteosarcoma cell line SaOS-2. Clin. Cancer Res. 12, 1420–1430 (2006).
Zhang, X. Y. Interactions between cancer cells and bone microenvironment promote bone metastasis in prostate cancer. Cancer Commun. 39, 76 (2019).
Cohen, P., Peehl, D. M., Graves, H. C. & Rosenfeld, R. G. Biological effects of prostate specific antigen as an insulin-like growth factor binding protein-3 protease. J. Endocrinol. 142, 407–415 (1994).
Cramer, S. D., Chen, Z. & Peehl, D. M. Prostate specific antigen cleaves parathyroid hormone-related protein in the PTH-like domain: inactivation of PTHrP-stimulated cAMP accumulation in mouse osteoblasts. J. Urol. 156, 526–531 (1996).
Iwamura, M., Hellman, J., Cockett, A. T., Lilja, H. & Gershagen, S. Alteration of the hormonal bioactivity of parathyroid hormone-related protein (PTHrP) as a result of limited proteolysis by prostate-specific antigen. Urology 48, 317–325 (1996).
Martin, T. J. Parathyroid hormone-related protein, its regulation of cartilage and bone development, and role in treating bone diseases. Physiol. Rev. 96, 831–871 (2016).
Frieling, J. S. & Lynch, C. C. Proteolytic regulation of parathyroid hormone-related protein: functional implications for skeletal malignancy. Int. J. Mol. Sci. 20, 2814 (2019).
Josson, S., Matsuoka, Y., Chung, L. W., Zhau, H. E. & Wang, R. Tumor–stroma co-evolution in prostate cancer progression and metastasis. Semin. Cell Dev. Biol. 21, 26–32 (2010).
Drucker, K. L. et al. Clinical significance and novel mechanism of action of kallikrein 6 in glioblastoma. Neuro Oncol. 15, 305–318 (2013).
Kim, T. W. et al. Kallikrein-related peptidase 6 induces chemotherapeutic resistance by attenuating auranofin-induced cell death through activation of autophagy in gastric cancer. Oncotarget 7, 85332–85348 (2016).
Jin, Y. et al. Molecular circuit involving KLK4 integrates androgen and mTOR signaling in prostate cancer. Proc. Natl Acad. Sci. USA 110, E2572 (2013).
Sotiropoulou, G. & Pampalakis, G. Targeting the kallikrein-related peptidases for drug development. Trends Pharmacol. Sci. 33, 623–634 (2012).
Goettig, P., Magdolen, V. & Brandstetter, H. Natural and synthetic inhibitors of kallikrein-related peptidases (KLKs). Biochimie 92, 1546–1567 (2010).
Mavridis, K., Avgeris, M. & Scorilas, A. Targeting kallikrein-related peptidases in prostate cancer. Expert Opin. Ther. Targets 18, 365–383 (2014).
Cereda, V., Formica, V., Menghi, A., Pellicori, S. & Roselli, M. Kallikrein-related peptidases targeted therapies in prostate cancer: perspectives and challenges. Expert Opin. Investig. Drugs 24, 929–947 (2015).
Oikonomopoulou, K. et al. Functional proteomics of kallikrein-related peptidases in ovarian cancer ascites fluid. Biol. Chem. 391, 381–390 (2010).
Sauer, A. K. et al. Zinc deficiency in men over 50 and its implications in prostate disorders. Front. Oncol. 10, 1293 (2020).
Hekim, C. et al. Novel peptide inhibitors of human kallikrein 2. J. Biol. Chem. 281, 12555–12560 (2006).
Cloutier, S. M. et al. Development of recombinant inhibitors specific to human kallikrein 2 using phage-display selected substrates. Eur. J. Biochem. 271, 607–613 (2004).
Pakkala, M. et al. Activity and stability of human kallikrein-2-specific linear and cyclic peptide inhibitors. J. Pept. Sci. 13, 348–353 (2007).
LeBeau, A. M., Banerjee, S. R., Pomper, M. G., Mease, R. C. & Denmeade, S. R. Optimization of peptide-based inhibitors of prostate-specific antigen (PSA) as targeted imaging agents for prostate cancer. Bioorgan Med. Chem. 17, 4888–4893 (2009).
Deperthes, D. & Kundig, C. Kallikrein-related peptidases as pharmaceutical targets. Trends Pharmacol. Sci. 33, 623–634 (2012).
Riley, B. T. et al. KLK4 inhibition by cyclic and acyclic peptides: structural and dynamical insights into standard-mechanism protease inhibitors. Biochemistry 58, 2524–2533 (2019).
Riley, B. T. et al. Direct and indirect mechanisms of KLK4 inhibition revealed by structure and dynamics. Sci. Rep. 6, 35385 (2016). Together with Koistinen et al. (2008), Hekim et al. (2006), Cloutier et al. (2004) and Pakkala et al. (2007), this work highlights the utility of peptides in inhibiting specific kallikreins.
de Veer, S. J. et al. Mechanism-based selection of a potent kallikrein-related peptidase 7 inhibitor from a versatile library based on the sunflower trypsin inhibitor SFTI-1. Biopolymers 100, 510–518 (2013).
de Veer, S. J. et al. Selective substrates and inhibitors for kallikrein-related peptidase 7 (KLK7) shed light on KLK proteolytic activity in the stratum corneum. J. Invest. Dermatol. 137, 430–439 (2017).
Sananes, A. et al. A potent, proteolysis-resistant inhibitor of kallikrein-related peptidase 6 (KLK6) for cancer therapy, developed by combinatorial engineering. J. Biol. Chem. 293, 12663–12680 (2018).
Candido, J. B. et al. Kallikrein-related peptidase 6 is associated with the tumour microenvironment of pancreatic ductal adenocarcinoma. Cancers (Basel) 13, 3969 (2021).
Kostova, M. B., Rosen, D. M., Chen, Y., Mease, R. C. & Denmeade, S. R. Structural optimization, biological evaluation, and application of peptidomimetic prostate specific antigen inhibitors. J. Med.Chem. 56, 4224–4235 (2013).
LeBeau, A. M., Singh, P., Isaacs, J. T. & Denmeade, S. R. Potent and selective peptidyl boronic acid inhibitors of the serine protease prostate-specific antigen. Chem. Biol. 15, 665–674 (2008).
Lovell, S. et al. A suite of activity-based probes to dissect the KLK activome in drug-resistant prostate cancer. J. Am. Chem. Soc. 143, 8911–8924 (2021).
Pereira, S. G. T. et al. Intracellular activation of a prostate specific antigen-cleavable doxorubicin prodrug: a key feature toward prodrug-nanomedicine design. Mol. Pharm. 16, 1573–1585 (2019).
DiPaola, R. S. et al. Characterization of a novel prostate-specific antigen-activated peptide-doxorubicin conjugate in patients with prostate cancer. J. Clin. Oncol. 20, 1874–1879 (2002).
Barve, A., Jain, A., Liu, H., Jin, W. & Cheng, K. An enzyme-responsive conjugate improves the delivery of a PI3K inhibitor to prostate cancer. Nanomedicine 12, 2373–2381 (2016).
Li, B. et al. Synergistic tumor growth-inhibitory effect of the prostate-specific antigen-activated fusion peptide BSD352 for prostate cancer therapy. Anticancer. Drugs 22, 213–222 (2011).
Denmeade, S. R. et al. Prostate-specific antigen-activated thapsigargin prodrug as targeted therapy for prostate cancer. J. Natl Cancer Inst. 95, 990–1000 (2003).
Janssen, S. et al. Pharmacokinetics, biodistribution, and antitumor efficacy of a human glandular kallikrein 2 (hK2)-activated thapsigargin prodrug. Prostate 66, 358–368 (2006).
Aloysius, H. & Hu, L. Improving the specificity of the prostate-specific antigen substrate Glutaryl-Hyp-Ala-Ser-Chg-Gln as a promoiety. Chem. Biol. Drug Des. 86, 837–848 (2015).
Aloysius, H. & Hu, L. Q. Synthesis and evaluation of new peptide-linked doxorubicin conjugates as prodrugs activated by prostate-specific antigen. Med. Chem. Res. 29, 1280–1299 (2020).
Williams, S. A. et al. A prostate-specific antigen-activated channel-forming toxin as therapy for prostatic disease. J. Natl Cancer Inst. 99, 376–385 (2007).
Rogers, O. C. et al. Microparticle encapsulation of a prostate-targeted biologic for the treatment of liver metastases in a preclinical model of castration-resistant prostate cancer. Mol. Cancer Ther. 19, 2353–2362 (2020).
Timmermand, O. V. et al. Preclinical imaging of kallikrein-related peptidase 2 (hK2) in prostate cancer with a 111In-radiolabelled monoclonal antibody, 11B6. EJNMMI Res. 4, 51 (2014). This study presents a novel theranostic approach targeting KLK2 for prostate cancer.
Evans-Axelsson, S. et al. Targeting free prostate-specific antigen for in vivo imaging of prostate cancer using a monoclonal antibody specific for unique epitopes accessible on free prostate-specific antigen alone. Cancer Biother Radiopharm. 27, 243–251 (2012).
Du, D., Fu, H. J., Ren, W. W., Li, X. L. & Guo, L. H. PSA targeted dual-modality manganese oxide–mesoporous silica nanoparticles for prostate cancer imaging. Biomed. Pharmacother. 121, 109614 (2020).
McDevitt, M. R. et al. Feed-forward alpha particle radiotherapy ablates androgen receptor-addicted prostate cancer. Nat. Commun. 9, 1629 (2018).
Veach, D. R. et al. PSA-targeted α-, β-, and positron-emitting immunotheranostics in murine prostate cancer models and nonhuman primates. Clin. Cancer Res. 27, 2050–2060 (2021).
Timmermand, O. V., Larsson, E., Ulmert, D., Tran, T. A. & Strand, S. E. Radioimmunotherapy of prostate cancer targeting human kallikrein-related peptidase 2. EJNMMI Res. 6, 27 (2016).
Abdul Sater, H. et al. Neoadjuvant PROSTVAC prior to radical prostatectomy enhances T-cell infiltration into the tumor immune microenvironment in men with prostate cancer. J Immunother Cancer 8, e000655 (2020).
Tang, L. et al. Long non-coding RNA LINC01314 represses cell migration, invasion, and angiogenesis in gastric cancer via the Wnt/β-catenin signaling pathway by down-regulating KLK4. Cancer Cell Int. 19, 94 (2019).
Zhu, S. P., Wang, J. Y., Wang, X. G. & Zhao, J. P. Long intergenic non-protein coding RNA 00858 functions as a competing endogenous RNA for miR-422a to facilitate the cell growth in non-small cell lung cancer. Aging 9, 475–486 (2017).
Matin, F. et al. microRNA-3162-5p-mediated crosstalk between kallikrein family members including prostate-specific antigen in prostate cancer. Clin. Chem. 65, 771–780 (2019).
Fields, G. B. The rebirth of matrix metalloproteinase inhibitors: moving beyond the dogma. Cells 8, 984 (2019).
Michael, I. P. et al. Human tissue kallikrein 5 is a member of a proteolytic cascade pathway involved in seminal clot liquefaction and potentially in prostate cancer progression. J. Biol. Chem. 281, 12743–12750 (2006).
Du, J. P. et al. Kallikrein-related peptidase 7 is a potential target for the treatment of pancreatic cancer. Oncotarget 9, 12894–12906 (2018).
Prezas, P. et al. Overexpression of the human tissue kallikrein genes KLK4, 5, 6, and 7 increases the malignant phenotype of ovarian cancer cells. Biol. Chem. 387, 807–811 (2006).
Loessner, D., Little, J. P., Pettet, G. J. & Hutmacher, D. W. A multiscale road map of cancer spheroids — incorporating experimental and mathematical modelling to understand cancer progression. J. Cell Sci. 126, 2761–2771 (2013).
Wang, P. et al. Kallikrein-related peptidases 4, 5, 6 and 7 regulate tumour-associated factors in serous ovarian cancer. Br. J. Cancer 119, 1–9 (2018).
Oikonomopoulou, K., Diamandis, E. P. & Hollenberg, M. D. Kallikrein-related peptidases: proteolysis and signaling in cancer, the new frontier. Biol. Chem. 391, 299–310 (2010).
Mize, G. J., Wang, W. & Takayama, T. K. Prostate-specific kallikreins-2 and -4 enhance the proliferation of DU-145 prostate cancer cells through protease-activated receptors-1 and -2. Mol. Cancer Res. 6, 1043–1051 (2008).
Shah, M. R. et al. Direct intra-tumoral injection of zinc-acetate halts tumor growth in a xenograft model of prostate cancer. J. Exp. Clin. Cancer Res. 28, 84 (2009).
Elhilali, M. M. et al. Prospective, randomized, double-blind, vehicle controlled, multicenter phase IIb clinical trial of the pore forming protein PRX302 for targeted treatment of symptomatic benign prostatic hyperplasia. J. Urol. 189, 1421–1426 (2013).
Karan, D. et al. Dual antigen target-based immunotherapy for prostate cancer eliminates the growth of established tumors in mice. Immunotherapy 3, 735–746 (2011).
Gamat-Huber, M. et al. Treatment combinations with DNA vaccines for the treatment of metastatic castration-resistant prostate cancer (mCRPC). Cancers (Basel) 12, 2831 (2020).
Sinha, A. A., Quast, B. J., Reddy, P. K., Elson, M. K. & Wilson, M. J. Intravenous injection of an immunoconjugate (anti-PSA-IgG conjugated to 5-fluoro-2′-deoxyuridine) selectively inhibits cell proliferation and induces cell death in human prostate cancer cell tumors grown in nude mice. Anticancer. Res. 19, 893–902 (1999).
Acknowledgements
This work was supported by a Translational Research Institute spore grant to J.B., J.C. and S.S., a Department of Defense Idea Development Award to J.B. and a National Health and Medical Research Council (NHMRC) Principal Research Fellowship to J.C. T.K. is a National Breast Cancer Foundation Fellow. The authors thank S. Seth for contributing to formation of the tables. S.S. is supported by an Advance Queensland Industry Early Career Research Fellowship. J.B. is supported by an Advance Queensland Industry Mid Career Research Fellowship. J.C. is an Emeritus Distinguished Professor at Queensland University of Technology.
Author information
Authors and Affiliations
Contributions
S.S. researched the data and drafted the article. T.K. contributed the figures. All authors made substantial contribution to the discussion of content, and reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Cancer thanks Johann de Bono, Hannu Koistinen, who co-reviewed the manuscript with Ruusu-Maaria Kovanen, and Andreas Scorilas for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Glossary
- Zymogen forms
-
Proteolytically inactive precursor forms of a protease.
- Tumour microenvironment
-
(TME). The area surrounding a tumour, in which other cell types, molecules and components such as blood vessels, immune cells, (myo)fibroblasts, cancer-associated fibroblasts (CAFs), tumour-associated macrophages (TAMs), bone marrow-derived stromal cells, signalling molecules and extracellular matrix (ECM) also are also found and considered to be altered owing to this changed cancer-associated environment.
- Extracellular matrix
-
(ECM). The structural matrix that consists of a multitude of proteins that provide structural support and organizational architecture to tissue.
- Cancer-associated fibroblasts
-
(CAFs). Activated fibroblasts associated with malignant tumours that have diverse functions such as creating extracellular matrix (ECM) and metabolic and immune reprogramming of the tumour microenvironment.
- Desmoplastic stroma
-
Tumour stroma consisting of dense fibrous or connective tissue.
- Meprins
-
Meprin proteinases are members of the metzincin superfamily and are proteases involved in tissue homeostasis, extracellular matrix (ECM) remodelling and immunological processes.
- Angiogenesis
-
Formation of new blood vessels.
- Lymphangiogenesis
-
Formation of new lymphatic vessels from pre-existing lymphatic vessels.
Rights and permissions
About this article
Cite this article
Srinivasan, S., Kryza, T., Batra, J. et al. Remodelling of the tumour microenvironment by the kallikrein-related peptidases. Nat Rev Cancer 22, 223–238 (2022). https://doi.org/10.1038/s41568-021-00436-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41568-021-00436-z
This article is cited by
-
Recent advances in the discovery and development of drugs targeting the kallikrein-kinin system
Journal of Translational Medicine (2024)
-
Tumor-derived KLK8 predicts inferior survival and promotes an immune-suppressive tumor microenvironment in lung squamous cell carcinoma
BMC Pulmonary Medicine (2024)
-
CSChighE-cadherinlow immunohistochemistry panel predicts poor prognosis in oral squamous cell carcinoma
Scientific Reports (2024)
-
The Plasma Kallikrein-Kinin System: A Hematological Target for Environmental Contaminants
Current Pollution Reports (2024)
-
Biomaterial-based platforms for tumour tissue engineering
Nature Reviews Materials (2023)