Abstract
Focal adhesion kinase (FAK) is both a non-receptor tyrosine kinase and an adaptor protein that primarily regulates adhesion signalling and cell migration, but FAK can also promote cell survival in response to stress. FAK is commonly overexpressed in cancer and is considered a high-value druggable target, with multiple FAK inhibitors currently in development. Evidence suggests that in the clinical setting, FAK targeting will be most effective in combination with other agents so as to reverse failure of chemotherapies or targeted therapies and enhance efficacy of immune-based treatments of solid tumours. Here, we discuss the recent preclinical evidence that implicates FAK in anticancer therapeutic resistance, leading to the view that FAK inhibitors will have their greatest utility as combination therapies in selected patient populations.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Lee, B. Y., Timpson, P., Horvath, L. G. & Daly, R. J. FAK signaling in human cancer as a target for therapeutics. Pharmacol. Ther. 146, 132–149 (2015).
Sulzmaier, F. J., Jean, C. & Schlaepfer, D. D. FAK in cancer: mechanistic findings and clinical applications. Nat. Rev. Cancer 14, 598–610 (2014).
Lietha, D. et al. Structural basis for the autoinhibition of focal adhesion kinase. Cell 129, 1177–1187 (2007).
Acebron, I. et al. Structural basis of focal adhesion kinase activation on lipid membranes. EMBO J. 39, e104743 (2020).
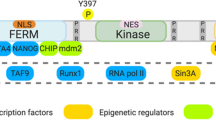
Frame, M. C., Patel, H., Serrels, B., Lietha, D. & Eck, M. J. The FERM domain: organizing the structure and function of FAK. Nat. Rev. Mol. Cell Biol. 11, 802–814 (2010).
Sanchez-Vega, F. et al. Oncogenic signaling pathways in the Cancer Genome Atlas. Cell 173, 321–337.e10 (2018).
Kaveh, F. et al. A systematic comparison of copy number alterations in four types of female cancer. BMC Cancer 16, 913 (2016).
Feng, X. et al. A platform of synthetic lethal gene interaction networks reveals that the GNAQ uveal melanoma oncogene controls the hippo pathway through FAK. Cancer Cell 35, 457–472 (2019). This study establishes FAK as a therapeutic target for the treatment of uveal melanoma with oncogenic mutations in GNAQ heterotrimeric G proteins.
Kim, Y. H. et al. FAK-copy-gain is a predictive marker for sensitivity to FAK inhibition in breast cancer. Cancers 11, 1288 (2019).
Agochiya, M. et al. Increased dosage and amplification of the focal adhesion kinase gene in human cancer cells. Oncogene 18, 5646–5653 (1999).
Gorringe, K. L. et al. Copy number analysis identifies novel interactions between genomic loci in ovarian cancer. PLoS ONE 5, e11408 (2010).
Ramakrishna, M. et al. Identification of candidate growth promoting genes in ovarian cancer through integrated copy number and expression analysis. PLoS ONE 5, e9983 (2010).
Goode, E. L. et al. A genome-wide association study identifies susceptibility loci for ovarian cancer at 2q31 and 8q24. Nat. Genet. 42, 874–879 (2010).
Zhang, H. et al. Gain-of-function RHOA mutations promote focal adhesion kinase activation and dependency in diffuse gastric cancer. Cancer Discov. 10, 288–305 (2020).
Lim, S. T. et al. Nuclear FAK promotes cell proliferation and survival through FERM-enhanced p53 degradation. Mol. Cell 29, 9–22 (2008).
Serrels, A. et al. Nuclear FAK controls chemokine transcription, Tregs, and evasion of anti-tumor immunity. Cell 163, 160–173 (2015). This study is the first to show that nuclear FAK promotes a suppressive immune environment.
Naser, R., Aldehaiman, A., Diaz-Galicia, E. & Arold, S. T. Endogenous control mechanisms of FAK and PYK2 and their relevance to cancer development. Cancers 10, 196 (2018).
Gao, C. et al. FAK/PYK2 promotes the Wnt/β-catenin pathway and intestinal tumorigenesis by phosphorylating GSK3β. eLife 4, e10072 (2015).
Lulo, J., Yuzawa, S. & Schlessinger, J. Crystal structures of free and ligand-bound focal adhesion targeting domain of Pyk2. Biochem. Biophys. Res. Commun. 383, 347–352 (2009).
Thul, P. J. et al. A subcellular map of the human proteome. Science 356, eaal3321 (2017).
Zhao, J., Zheng, C. & Guan, J. Pyk2 and FAK differentially regulate progression of the cell cycle. J. Cell Sci. 113, 3063–3072 (2000).
Lim, Y. et al. PyK2 and FAK connections to p190Rho guanine nucleotide exchange factor regulate RhoA activity, focal adhesion formation, and cell motility. J. Cell Biol. 180, 187–203 (2008).
Weis, S. M. et al. Compensatory role for Pyk2 during angiogenesis in adult mice lacking endothelial cell FAK. J. Cell Biol. 181, 43–50 (2008).
Fan, H. & Guan, J. L. Compensatory function of Pyk2 protein in the promotion of focal adhesion kinase (FAK)-null mammary cancer stem cell tumorigenicity and metastatic activity. J. Biol. Chem. 286, 18573–18582 (2011).
Slack-Davis, J. K. et al. Cellular characterization of a novel focal adhesion kinase inhibitor. J. Biol. Chem. 282, 14845–14852 (2007).
Hirt, U. A. et al. Efficacy of the highly selective focal adhesion kinase inhibitor BI 853520 in adenocarcinoma xenograft models is linked to a mesenchymal tumor phenotype. Oncogenesis 7, 21 (2018).
Klaeger, S. et al. The target landscape of clinical kinase drugs. Science 358, eaan4368 (2017).
Roberts, W. G. et al. Antitumor activity and pharmacology of a selective focal adhesion kinase inhibitor, PF-562,271. Cancer Res. 68, 1935–1944 (2008).
McLean, G. W. et al. Specific deletion of focal adhesion kinase suppresses tumor formation and blocks malignant progression. Genes Dev. 18, 2998–3003 (2004).
Ashton, G. H. et al. Focal adhesion kinase is required for intestinal regeneration and tumorigenesis downstream of Wnt/c-Myc signaling. Dev. Cell 19, 259–269 (2010).
Lahlou, H. et al. Mammary epithelial-specific disruption of the focal adhesion kinase blocks mammary tumor progression. Proc. Natl Acad. Sci. USA 104, 20302–20307 (2007).
Luo, M. et al. Mammary epithelial-specific ablation of the focal adhesion kinase suppresses mammary tumorigenesis by affecting mammary cancer stem/progenitor cells. Cancer Res. 69, 466–474 (2009).
Slack-Davis, J. K., Hershey, E. D., Theodorescu, D., Frierson, H. F. & Parsons, J. T. Differential requirement for focal adhesion kinase signaling in cancer progression in the transgenic adenocarcinoma of mouse prostate model. Mol. Cancer Ther. 8, 2470–2477 (2009).
Soria, J. C. et al. A phase I, pharmacokinetic and pharmacodynamic study of GSK2256098, a focal adhesion kinase inhibitor, in patients with advanced solid tumors. Ann. Oncol. 27, 2268–2274 (2016).
Shimizu, T. et al. A first-in-Asian phase 1 study to evaluate safety, pharmacokinetics and clinical activity of VS-6063, a focal adhesion kinase (FAK) inhibitor in Japanese patients with advanced solid tumors. Cancer Chemother. Pharmacol. 77, 997–1003 (2016).
Jones, S. F. et al. A phase I study of VS-6063, a second-generation focal adhesion kinase inhibitor, in patients with advanced solid tumors. Invest. New Drugs 33, 1100–1107 (2015).
Gerber, D. E. et al. Phase 2 study of the focal adhesion kinase inhibitor defactinib (VS-6063) in previously treated advanced KRAS mutant non-small cell lung cancer. Lung Cancer 139, 60–67 (2020).
Chapman, P. B. et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N. Engl. J. Med. 364, 2507–2516 (2011).
Luke, J. J., Flaherty, K. T., Ribas, A. & Long, G. V. Targeted agents and immunotherapies: optimizing outcomes in melanoma. Nat. Rev. Clin. Oncol. 14, 463–482 (2017).
Nazarian, R. et al. Melanomas acquire resistance to B-RAF(V600E) inhibition by RTK or N-RAS upregulation. Nature 468, 973–977 (2010).
Diaz Osterman, C. J. et al. FAK activity sustains intrinsic and acquired ovarian cancer resistance to platinum chemotherapy. eLife 8, e47327 (2019). This study demonstrates that WNT–β-catenin signalling activated by anchorage-independent activation of FAK supports platinum chemoresistance in ovarian cancer.
Jiang, H. et al. Targeting focal adhesion kinase renders pancreatic cancers responsive to checkpoint immunotherapy. Nat. Med. 22, 851–860 (2016). This study demonstrates that FAK inhibition in PDAC tumours can overcome an immunosuppressive fibrotic tumour microenvironment and render tumours responsive to immunotherapy and chemotherapy.
Skinner, H. D. et al. Proteomic profiling identifies PTK2/FAK as a driver of radioresistance in HPV-negative head and neck cancer. Clin. Cancer Res. 22, 4643–4650 (2016).
Tang, K.-J. et al. Focal adhesion kinase regulates the DNA damage response and its inhibition radiosensitizes mutant KRAS lung cancer. Clin. Cancer Res. 22, 5851–5863 (2016).
Williams, K. E., Bundred, N. J., Landberg, G., Clarke, R. B. & Farnie, G. Focal adhesion kinase and Wnt signaling regulate human ductal carcinoma in situ stem cell activity and response to radiotherapy. Stem Cell 33, 327–341 (2015).
Chen, G. et al. Wnt/beta-catenin pathway activation mediates adaptive resistance to BRAF inhibition in colorectal cancer. Mol. Cancer Ther. 17, 806–813 (2018).
Fallahi-Sichani, M. et al. Adaptive resistance of melanoma cells to RAF inhibition via reversible induction of a slowly dividing de-differentiated state. Mol. Syst. Biol. 13, 905 (2017).
Hirata, E. et al. Intravital imaging reveals how BRAF inhibition generates drug-tolerant microenvironments with high integrin beta1/FAK signaling. Cancer Cell 27, 574–588 (2015). This study uses intravital imaging to identify how molecularly targeted therapy can activate the tumour stroma to rapidly promote a drug-tolerant environment and protect melanoma cells via activation of FAK from cell death.
Canel, M. et al. T-cell co-stimulation in combination with targeting FAK drives enhanced anti-tumor immunity. eLife 9, e48092 (2020).
Serrels, B. et al. IL-33 and ST2 mediate FAK-dependent antitumor immune evasion through transcriptional networks. Sci. Signal. 10, eaan8355 (2017).
Dawson, J. C. et al. A synergistic anticancer FAK and HDAC inhibitor combination discovered by a novel chemical-genetic high-content phenotypic screen. Mol. Cancer Ther. 19, 637–649 (2020).
Tavora, B. et al. Endothelial-cell FAK targeting sensitizes tumours to DNA-damaging therapy. Nature 514, 112–116 (2014).
Wu, H. J., Hao, M., Yeo, S. K. & Guan, J. L. FAK signaling in cancer-associated fibroblasts promotes breast cancer cell migration and metastasis by exosomal miRNAs-mediated intercellular communication. Oncogene 39, 2539–2549 (2020).
Zaghdoudi, S. et al. FAK activity in cancer-associated fibroblasts is a prognostic marker and a druggable key metastatic player in pancreatic cancer. EMBO Mol. Med. 12, e12010 (2020). This study identifies stromal activation of FAK as an independent prognostic marker for disease-free survival in pancreatic cancer and highlights the potential of targeting the tumour stroma for the treatment of cancer.
Tang, J. et al. Drug target commons: a community effort to build a consensus knowledge base for drug-target interactions. Cell Chem. Biol. 25, 224–229 (2018).
Patel, M. R. et al. Phase 1/1b study of the FAK inhibitor defactinib (VS-6063) in combination with weekly paclitaxel for advanced ovarian cancer. J. Clin. Oncol. 32, 5521–5521 (2014).
Aung, K. L. et al. A phase II trial of GSK2256098 and trametinib in patients with advanced pancreatic ductal adenocarcinoma (PDAC) (MOBILITY-002 Trial, NCT02428270). J. Clin. Oncol. 36, 409–409 (2018).
Mak, G. et al. A phase Ib dose-finding, pharmacokinetic study of the focal adhesion kinase inhibitor GSK2256098 and trametinib in patients with advanced solid tumours. Br. J. Cancer 120, 975–981 (2019).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT04625270 (2020).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT04620330 (2020).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT04331041 (2020).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT04109456 (2020).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT04201145 (2020).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT03875820 (2020).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT03727880 (2020).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT03287271 (2020).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT02758587 (2018).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT02546531 (2020).
Siegel, R. L., Miller, K. D. & Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 70, 7–30 (2020).
Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature 474, 609–615 (2011).
Ducie, J. et al. Molecular analysis of high-grade serous ovarian carcinoma with and without associated serous tubal intra-epithelial carcinoma. Nat. Commun. 8, 990 (2017).
Matulonis, U. A. et al. Ovarian cancer. Nat. Rev. Dis. Primers 2, 16061 (2016).
Lee, J. M., Minasian, L. & Kohn, E. C. New strategies in ovarian cancer treatment. Cancer 125 (Suppl. 24), 4623–4629 (2019).
Laklai, H. et al. Genotype tunes pancreatic ductal adenocarcinoma tissue tension to induce matricellular fibrosis and tumor progression. Nat. Med. 22, 497–505 (2016).
Kang, Y. et al. Role of focal adhesion kinase in regulating YB-1-mediated paclitaxel resistance in ovarian cancer. J. Natl Cancer Inst. 105, 1485–1495 (2013).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT01778803 (2017).
Walton, J. et al. CRISPR/Cas9-Mediated Trp53 and Brca2 knockout to generate improved murine models of ovarian high-grade serous carcinoma. Cancer Res. 76, 6118–6129 (2016).
Ward, K. K. et al. Inhibition of focal adhesion kinase (FAK) activity prevents anchorage-independent ovarian carcinoma cell growth and tumor progression. Clin. Exp. Metastasis 30, 579–594 (2013).
Nagaraj, A. B. et al. Critical role of Wnt/beta-catenin signaling in driving epithelial ovarian cancer platinum resistance. Oncotarget 6, 23720–23734 (2015).
Kolev, V. N. et al. Inhibition of FAK kinase activity preferentially targets cancer stem cells. Oncotarget 8, 51733–51747 (2017).
Kazi, J. U., Kabir, N. N. & Ronnstrand, L. Brain-expressed X-linked (BEX) proteins in human cancers. Biochim. Biophys. Acta 1856, 226–233 (2015).
Delaney, J. R. et al. Haploinsufficiency networks identify targetable patterns of allelic deficiency in low mutation ovarian cancer. Nat. Commun. 8, 14423 (2017).
Lengyel, E., Makowski, L., DiGiovanni, J. & Kolonin, M. G. Cancer as a matter of fat: the crosstalk between adipose tissue and tumors. Trends Cancer 4, 374–384 (2018).
Jana, S. et al. SOX9: The master regulator of cell fate in breast cancer. Biochem. Pharmacol. 174, 113789 (2020).
Kang, Y. et al. Adrenergic stimulation of DUSP1 impairs chemotherapy response in ovarian cancer. Clin. Cancer Res. 22, 1713–1724 (2016).
Ma, Q. et al. Super-enhancer redistribution as a mechanism of broad gene dysregulation in repeatedly drug-treated cancer cells. Cell Rep. 31, 107532 (2020).
Hobbs, G. A., Der, C. J. & Rossman, K. L. RAS isoforms and mutations in cancer at a glance. J. Cell Sci. 129, 1287–1292 (2016).
Kim, M. H. et al. Actin remodeling confers BRAF inhibitor resistance to melanoma cells through YAP/TAZ activation. EMBO J. 35, 462–478 (2016).
Shinde, R. et al. Abstract CT143: phase I study of the combination of a RAF-MEK inhibitor CH5126766 and FAK inhibitor defactinib in an intermittent dosing schedule with expansions in KRAS mutant cancers. Cancer Res. 80, CT143 (2020).
Verastem Oncology. Addressing RAS pathway blockade and resistance. Verastem https://investor.verastem.com/static-files/93835009-c4b6-4818-9075-16cb64237930 (2020).
Zheng, Y. et al. FAK phosphorylation by ERK primes ras-induced tyrosine dephosphorylation of FAK mediated by PIN1 and PTP-PEST. Mol. Cell 35, 11–25 (2009).
Horton, E. R. et al. Definition of a consensus integrin adhesome and its dynamics during adhesion complex assembly and disassembly. Nat. Cell Biol. 17, 1577–1587 (2015). This study defines a core machinery that is required for integrin-based focal adhesions in cells attached to fibronectin.
Kim, N. G. & Gumbiner, B. M. Adhesion to fibronectin regulates Hippo signaling via the FAK-Src-PI3K pathway. J. Cell Biol. 210, 503–515 (2015).
Zanconato, F., Cordenonsi, M. & Piccolo, S. YAP and TAZ: a signalling hub of the tumour microenvironment. Nat. Rev. Cancer 19, 454–464 (2019).
Zanconato, F., Cordenonsi, M. & Piccolo, S. YAP/TAZ at the roots of cancer. Cancer Cell 29, 783–803 (2016).
Zanconato, F., Battilana, G., Cordenonsi, M. & Piccolo, S. YAP/TAZ as therapeutic targets in cancer. Curr. Opin. Pharmacol. 29, 26–33 (2016).
Lin, L. et al. The Hippo effector YAP promotes resistance to RAF- and MEK-targeted cancer therapies. Nat. Genet. 47, 250–256 (2015).
Li, H. et al. YAP/TAZ activation drives uveal melanoma initiation and progression. Cell Rep. 29, 3200–3211 (2019).
Paradis, J. S. et al. Synthetic lethal screens reveal co-targeting FAK and MEK as a multimodal precision therapy for GNAQ-driven uveal melanoma. Clin. Cancer Res. https://doi.org/10.1158/1078-0432.CCR-20-3363 (2021).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT04720417 (2021).
Elbediwy, A. et al. Integrin signalling regulates YAP and TAZ to control skin homeostasis. Development 143, 1674–1687 (2016).
Han, H. et al. Hippo signaling dysfunction induces cancer cell addiction to YAP. Oncogene 37, 6414–6424 (2018).
Suraweera, A., O’Byrne, K. J. & Richard, D. J. Combination therapy with histone deacetylase inhibitors (HDACi) for the treatment of cancer: achieving the full therapeutic potential of HDACi. Front. Oncol. 8, 92 (2018).
Liu-Chittenden, Y. et al. Genetic and pharmacological disruption of the TEAD-YAP complex suppresses the oncogenic activity of YAP. Genes Dev. 26, 1300–1305 (2012).
Zanconato, F. et al. Transcriptional addiction in cancer cells is mediated by YAP/TAZ through BRD4. Nat. Med. 24, 1599–1610 (2018).
Sharma, P., Hu-Lieskovan, S., Wargo, J. A. & Ribas, A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell 168, 707–723 (2017).
Linsley, P. S. et al. CTLA-4 is a second receptor for the B cell activation antigen B7. J. Exp. Med. 174, 561–569 (1991).
Linsley, P. S., Clark, E. A. & Ledbetter, J. A. T-cell antigen CD28 mediates adhesion with B cells by interacting with activation antigen B7/BB-1. Proc. Natl Acad. Sci. USA 87, 5031–5035 (1990).
Whitney, G. S. et al. Human T and B lymphocytes express a structurally conserved focal adhesion kinase, pp125FAK. DNA Cell Biol. 12, 823–830 (1993).
Chapman, N. M., Connolly, S. F., Reinl, E. L. & Houtman, J. C. Focal adhesion kinase negatively regulates Lck function downstream of the T cell antigen receptor. J. Immunol. 191, 6208–6221 (2013).
Courtney, A. H. et al. A phosphosite within the SH2 domain of lck regulates its activation by CD45. Mol. Cell 67, 498–511.e6 (2017).
Chapman, N. M. & Houtman, J. C. Functions of the FAK family kinases in T cells: beyond actin cytoskeletal rearrangement. Immunol. Res. 59, 23–34 (2014).
Raab, M. et al. LFA-1 activates focal adhesion kinases FAK1/PYK2 to generate LAT-GRB2-SKAP1 complexes that terminate T-cell conjugate formation. Nat. Commun. 8, 16001 (2017).
Gett, A. V., Sallusto, F., Lanzavecchia, A. & Geginat, J. T cell fitness determined by signal strength. Nat. Immunol. 4, 355–360 (2003).
Iezzi, G., Karjalainen, K. & Lanzavecchia, A. The duration of antigenic stimulation determines the fate of naive and effector T cells. Immunity 8, 89–95 (1998).
Wiemer, A. J. et al. The focal adhesion kinase inhibitor PF-562,271 impairs primary CD4+ T cell activation. Biochem. Pharmacol. 86, 770–781 (2013).
Batista, S. et al. Haematopoietic focal adhesion kinase deficiency alters haematopoietic homeostasis to drive tumour metastasis. Nat. Commun. 5, 5054 (2014).
Lechertier, T. et al. Pericyte FAK negatively regulates Gas6/Axl signalling to suppress tumour angiogenesis and tumour growth. Nat. Commun. 11, 2810 (2020).
Tavora, B. et al. Endothelial FAK is required for tumour angiogenesis. EMBO Mol. Med. 2, 516–528 (2010).
Pedrosa, A. R. et al. Tumor angiogenesis is differentially regulated by phosphorylation of endothelial cell focal adhesion kinase tyrosines-397 and -861. Cancer Res. 79, 4371–4386 (2019).
Jean, C. et al. Inhibition of endothelial FAK activity prevents tumor metastasis by enhancing barrier function. J. Cell Biol. 204, 247–263 (2014).
Demircioglu, F. et al. Cancer associated fibroblast FAK regulates malignant cell metabolism. Nat. Commun. 11, 1290 (2020).
Zhang, J. et al. Focal adhesion kinase-promoted tumor glucose metabolism is associated with a shift of mitochondrial respiration to glycolysis. Oncogene 35, 1926–1942 (2016).
Jiang, H. et al. Development of resistance to FAK inhibition in pancreatic cancer is linked to stromal depletion. Gut 69, 122–132 (2020).
Vennin, C. et al. Transient tissue priming via ROCK inhibition uncouples pancreatic cancer progression, sensitivity to chemotherapy, and metastasis. Sci. Transl Med. 9, eaai8504 (2017).
de Jonge, M. J. A. et al. Phase I study of BI 853520, an inhibitor of focal adhesion kinase, in patients with advanced or metastatic nonhematologic malignancies. Target. Oncol. 14, 43–55 (2019).
Schoenherr, C. et al. Ambra1 spatially regulates Src activity and Src/FAK-mediated cancer cell invasion via trafficking networks. eLife 6, e23172 (2017).
Serrels, B. et al. Focal adhesion kinase controls actin assembly via a FERM-mediated interaction with the Arp2/3 complex. Nat. Cell Biol. 9, 1046–1056 (2007).
Luo, M. et al. Distinct FAK activities determine progenitor and mammary stem cell characteristics. Cancer Res. 73, 5591–5602 (2013).
Gao, H. et al. FAK-targeting PROTAC as a chemical tool for the investigation of non-enzymatic FAK function in mice. Protein Cell 11, 534–539 (2020).
Cromm, P. M., Samarasinghe, K. T. G., Hines, J. & Crews, C. M. Addressing kinase-independent functions of Fak via PROTAC-mediated degradation. J. Am. Chem. Soc. 140, 17019–17026 (2018).
Popow, J. et al. Highly selective PTK2 proteolysis targeting chimeras to probe focal adhesion kinase scaffolding functions. J. Med. Chem. 62, 2508–2520 (2019).
Bantscheff, M. et al. Quantitative chemical proteomics reveals mechanisms of action of clinical ABL kinase inhibitors. Nat. Biotechnol. 25, 1035–1044 (2007).
Ma, H., Deacon, S. & Horiuchi, K. The challenge of selecting protein kinase assays for lead discovery optimization. Expert Opin. Drug Discov. 3, 607–621 (2008).
Frame, M. C. & Serrels, A. FAK to the rescue: activated stroma promotes a “safe haven” for BRAF-mutant melanoma cells by inducing FAK signaling. Cancer Cell 27, 429–431 (2015).
Stokes, J. B. et al. Inhibition of focal adhesion kinase by PF-562,271 inhibits the growth and metastasis of pancreatic cancer concomitant with altering the tumor microenvironment. Mol. Cancer Ther. 10, 2135–2145 (2011).
Guan, J. L. & Shalloway, D. Regulation of focal adhesion-associated protein tyrosine kinase by both cellular adhesion and oncogenic transformation. Nature 358, 690–692 (1992).
Schaller, M. D. et al. pp125FAK a structurally distinctive protein-tyrosine kinase associated with focal adhesions. Proc. Natl Acad. Sci. USA 89, 5192–5196 (1992).
Hanks, S. K., Calalb, M. B., Harper, M. C. & Patel, S. K. Focal adhesion protein-tyrosine kinase phosphorylated in response to cell attachment to fibronectin. Proc. Natl Acad. Sci. USA 89, 8487–8491 (1992).
Acknowledgements
M.C.F. is supported by a Cancer Research UK Programme Award (C157/A24837). J.C.D. is supported by a Cancer Research UK (C42454/A28596) and BrainTumour Charity (GN-000676) award. D.D.S. and D.G.S. are supported by funding from the US National Institutes of Health (R01 CA247562 and R01 CA254342). A.S. is supported by a Cancer Research UK Career Development Award (C39669/A25919).
Author information
Authors and Affiliations
Contributions
All authors made a substantial contribution to discussion of the content and writing the article. M.C.F., J.C.D., A.S., D.G.S. and D.D.S. researched data for the article. M.C.F., J.C.D., D.G.S. and D.D.S. reviewed and edited the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
A.S. has received research funding from Boehringer Ingelheim to work on IN10018 (then BI 853520) and is on the scientific advisory board of InxMed in relation to the development of IN10018. All other authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Cancer thanks J.-L. Guan, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
COSMIC database: https://cancer.sanger.ac.uk/cosmic
TCGA research network genomic data commons portal: https://portal.gdc.cancer.gov/
Glossary
- Focal adhesions
-
Points of cellular plasma membranes that link to extracellular matrix via transmembrane receptors, typically integrin heterodimers.
- Focal adhesion targeting (FAT) domain
-
A protein domain that is involved in the localization of focal adhesion kinase (FAK) to focal adhesions through interactions with other focal adhesion proteins.
- FERM (4.1 protein, ezrin, radixin and moesin) domain
-
A protein domain that is involved in localizing proteins to the plasma membrane.
- Heterotrimeric G proteins
-
A GTPase complex made up of three subunits, α, β and γ, that links transmembrane receptors to intracellular signalling pathways.
- Ras homologue family member A
-
(RHOA). A small GTPase primarily associated with regulating the actin cytoskeleton.
- Programmed cell death 1
-
(PD1). A protein expressed on the surface of cells that inhibits the activation of the immune system.
- Stromal cells
-
Connective tissue cells such as fibroblasts that support the other cells of that organ.
- Hippo pathway
-
A signalling pathway that controls organ size by regulating cell proliferation and apoptosis that can be dysregulated in cancer.
- Consensus integrin adhesome
-
The proteins that make up the core cell adhesion machinery of integrin adhesion complexes.
- Regulatory T cells
-
(Treg cells). A subpopulation of T cells that suppress the immune response.
- Cytotoxic T lymphocyte antigen 4
-
(CTLA4). A protein expressed by regulatory T cells that functions as an immune check point to inhibit the immune response.
- RHO-associated protein kinase
-
(ROCK). A serine/threonine kinase downstream effector of RAS homologue family member A (RHOA) involved in the formation of actin stress fibres.
Rights and permissions
About this article
Cite this article
Dawson, J.C., Serrels, A., Stupack, D.G. et al. Targeting FAK in anticancer combination therapies. Nat Rev Cancer 21, 313–324 (2021). https://doi.org/10.1038/s41568-021-00340-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41568-021-00340-6
This article is cited by
-
Matrix stiffness affects tumor-associated macrophage functional polarization and its potential in tumor therapy
Journal of Translational Medicine (2024)
-
Targeting of focal adhesion kinase enhances the immunogenic cell death of PEGylated liposome doxorubicin to optimize therapeutic responses of immune checkpoint blockade
Journal of Experimental & Clinical Cancer Research (2024)
-
AKT2S128/CCTαS315/319/323-positive cancer-associated fibroblasts (CAFs) mediate focal adhesion kinase (FAK) inhibitors resistance via secreting phosphatidylcholines (PCs)
Signal Transduction and Targeted Therapy (2024)
-
FAK-LINC01089 negative regulatory loop controls chemoresistance and progression of small cell lung cancer
Oncogene (2024)
-
Collagen I-induced VCAN/ERK signaling and PARP1/ZEB1-mediated metastasis facilitate OSBPL2 defect to promote colorectal cancer progression
Cell Death & Disease (2024)