Abstract
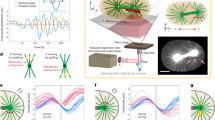
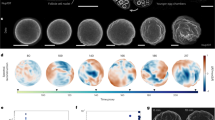
Life in complex systems, such as cities and organisms, comes to a standstill when global coordination of mass, energy and information flows is disrupted. Global coordination is no less important in single cells, especially in large oocytes and newly formed embryos, which commonly use fast fluid flows for dynamic reorganization of their cytoplasm. These cytoplasmic streaming flows have been proposed to spontaneously arise from hydrodynamic interactions among cortically anchored microtubules loaded with cargo-carrying molecular motors. Here, we combine modelling and simulation with live imaging to investigate such flows in the Drosophila oocyte. Using a fast, accurate and scalable numerical approach to investigate fluid–structure interactions of thousands of flexible fibres, we demonstrate the robust emergence and evolution of cell-spanning vortices—or twisters—in three-dimensional cellular geometries. These twister flows, dominated by a near-rigid-body rotation with secondary toroidal components, reproduce the variety of experimental observations. In cells, these flows are probably involved in rapid mixing and transport of ooplasmic components.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Simulational and experimental data sets generated during the current study are available from the corresponding author on reasonable request.
Code availability
A publicly available and elaborated version of the SkellySim codebase used to generate the simulations is available at https://github.com/flatironinstitute/SkellySim.
References
Corti, B. Osservazioni microscopiche sulla tremella e sulla circolazione del fluido in una pianta acquajuola (Rocchi, 1774).
Yi, K. et al. Dynamic maintenance of asymmetric meiotic spindle position through Arp2/3-complex-driven cytoplasmic streaming in mouse oocytes. Nat. Cell Biol. 13, 1252–1258 (2011).
Almonacid, M. et al. Active diffusion positions the nucleus in mouse oocytes. Nat. Cell Biol. 17, 470–479 (2015).
Deneke, V. E. et al. Self-organized nuclear positioning synchronizes the cell cycle in Drosophila embryos. Cell 177, 925–941 (2019).
Glotzer, J. B., Saffrich, R., Glotzer, M. & Ephrussi, A. Cytoplasmic flows localize injected oskar RNA in Drosophila oocytes. Curr. Biol. 7, 326–337 (1997).
van de Meent, J.-W., Tuval, I. & Goldstein, R. E. Nature’s microfluidic transporter: rotational cytoplasmic streaming at high Péclet numbers. Phys. Rev. Lett. 101, 178102 (2008).
Hird, S. N. & White, J. G. Cortical and cytoplasmic flow polarity in early embryonic cells of Caenorhabditis elegans. J. Cell Biol. 121, 1343–1355 (1993).
Emmons, S. et al. Cappuccino, a Drosophila maternal effect gene required for polarity of the egg and embryo, is related to the vertebrate limb deformity locus. Genes Dev. 9, 2482–2494 (1995).
Trong, P. K., Doerflinger, H., Dunkel, J., St Johnston, D. & Goldstein, R. E. Cortical microtubule nucleation can organise the cytoskeleton of Drosophila oocytes to define the anteroposterior axis. eLife 4, e06088 (2015).
Gross, P. et al. Guiding self-organized pattern formation in cell polarity establishment. Nat. Phys. 15, 293–300 (2019).
Goldstein, R. E. & van de Meent, J.-W. A physical perspective on cytoplasmic streaming. Interface Focus 5, 20150030 (2015).
Lu, W. & Gelfand, V. I. Go with the flow–bulk transport by molecular motors. J. Cell Sci. 136, jcs260300 (2023).
Shamipour, S., Caballero-Mancebo, S. & Heisenberg, C.-P. Cytoplasm’s got moves. Dev. Cell 56, 213–226 (2021).
Woodhouse, F. G. & Goldstein, R. E. Cytoplasmic streaming in plant cells emerges naturally by microfilament self-organization. Proc. Natl Acad. Sci. 110, 14132–14137 (2013).
Quinlan, M. E. Cytoplasmic streaming in the Drosophila oocyte. Annu. Rev. Cell Dev. Biol. 32, 173–195 (2016).
Becalska, A. N. & Gavis, E. R. Lighting up mRNA localization in Drosophila oogenesis. Development 136, 2493–2503 (2009).
Gutzeit, H. & Koppa, R. Time-lapse film analysis of cytoplasmic streaming during late oogenesis of Drosophila. Development 67, 101–111 (1982).
Monteith, C. E. et al. A mechanism for cytoplasmic streaming: kinesin-driven alignment of microtubules and fast fluid flows. Biophys. J. 110, 2053–2065 (2016).
Lu, W., Winding, M., Lakonishok, M., Wildonger, J. & Gelfand, V. I. Microtubule–microtubule sliding by kinesin-1 is essential for normal cytoplasmic streaming in Drosophila oocytes. Proc. Natl Acad. Sci. 113, E4995–E5004 (2016).
Palacios, I. M. & Johnston, D. S. Kinesin light chain-independent function of the kinesin heavy chain in cytoplasmic streaming and posterior localization in the Drosophila oocyte. Development 129, 5473–5485 (2002).
Serbus, L. R., Cha, B. J., Theurkauf, W. E. & Saxton, W. M. Dynein and the actin cytoskeleton control kinesin-driven cytoplasmic streaming in Drosophila oocytes. Development 132, 3743–52 (2005).
Ganguly, S., Williams, L. S., Palacios, I. M. & Goldstein, R. E. Cytoplasmic streaming in Drosophila oocytes varies with kinesin activity and correlates with the microtubule cytoskeleton architecture. Proc. Natl Acad. Sci. USA 109, 15109–15114 (2012).
Ravichandran, A. et al. Chronology of motor-mediated microtubule streaming. eLife 8, e39694 (2019).
Stein, D. B., De Canio, G., Lauga, E., Shelley, M. J. & Goldstein, R. E. Swirling instability of the microtubule cytoskeleton. Phys. Rev. Lett. 126, 028103 (2021).
Gittes, F., Mickey, B., Nettleton, J. & Howard, J. Flexural rigidity of microtubules and actin filaments measured from thermal fluctuations in shape. J. Cell Biol. 120, 923–934 (1993).
Shelley, M. J. The dynamics of microtubule/motor-protein assemblies in biology and physics. Annu. Rev. Fluid Mech. 48, 487–506 (2016).
Keller, J. B. & Rubinow, S. I. Slender-body theory for slow viscous flow. J. Fluid Mech. 75, 705–714 (1976).
Tornberg, A.-K. & Shelley, M. J. Simulating the dynamics and interactions of flexible fibers in stokes flows. J. Comput. Phys. 196, 8–40 (2004).
Nazockdast, E., Rahimian, A., Zorin, D. & Shelley, M. A fast platform for simulating semi-flexible fiber suspensions applied to cell mechanics. J. Comput. Phys. 329, 173–209 (2017).
Elgeti, J. & Gompper, G. Emergence of metachronal waves in cilia arrays. Proc. Natl Acad. Sci. USA 110, 4470–4475 (2013).
Chakrabarti, B., Fürthauer, S. & Shelley, M. J. A multiscale biophysical model gives quantized metachronal waves in a lattice of beating cilia. Proc. Natl Acad. Sci. USA 119, e2113539119 (2022).
SkellySim cellular dynamics package. GitHub https://github.com/flatironinstitute/SkellySim (2023).
Kanale, A. V., Ling, F., Guo, H., Fürthauer, S. & Kanso, E. Spontaneous phase coordination and fluid pumping in model ciliary carpets. Proc. Natl Acad. Sci. 119, e2214413119 (2022).
Theurkauf, W. E. Premature microtubule-dependent cytoplasmic streaming in cappuccino and spire mutant oocytes. Science 265, 2093–2096 (1994).
Dahlgaard, K., Raposo, A. A., Niccoli, T. & St Johnston, D. Capu and Spire assemble a cytoplasmic actin mesh that maintains microtubule organization in the Drosophila oocyte. Dev. Cell 13, 539–553 (2007).
Isele-Holder, R. E., Jäger, J., Saggiorato, G., Elgeti, J. & Gompper, G. Dynamics of self-propelled filaments pushing a load. Soft Matter 12, 8495–8505 (2016).
De Canio, G., Lauga, E. & Goldstein, R. E. Spontaneous oscillations of elastic filaments induced by molecular motors. J. R. Soc. Interface 14, 20170491 (2017).
Ling, F., Guo, H. & Kanso, E. Instability-driven oscillations of elastic microfilaments. J. R. Soc. Interface 15, 20180594 (2018).
Stone, H., Nadim, A. & Strogatz, S. H. Chaotic streamlines inside drops immersed in steady stokes flows. J. Fluid Mech. 232, 629–646 (1991).
Williams, L. S., Ganguly, S., Loiseau, P., Ng, B. F. & Palacios, I. M. The auto-inhibitory domain and ATP-independent microtubule-binding region of Kinesin heavy chain are major functional domains for transport in the Drosophila germline. Development 141, 176–186 (2014).
Brendza, K. M., Rose, D. J., Gilbert, S. P. & Saxton, W. M. Lethal kinesin mutations reveal amino acids important for atpase activation and structural coupling. J. Biol. Chem. 274, 31506–31514 (1999).
Loiseau, P., Davies, T., Williams, L. S., Mishima, M. & Palacios, I. M. Drosophila PAT1 is required for kinesin-1 to transport cargo and to maximize its motility. Development 137, 2763–2772 (2010).
Theurkauf, W. E., Smiley, S., Wong, M. L. & Alberts, B. M. Reorganization of the cytoskeleton during Drosophila oogenesis: implications for axis specification and intercellular transport. Development 115, 923–936 (1992).
Nashchekin, D., Fernandes, A. R. & St Johnston, D. Patronin/shot cortical foci assemble the noncentrosomal microtubule array that specifies the Drosophila anterior-posterior axis. Dev. Cell 38, 61–72 (2016).
Lu, W. et al. Competition between kinesin-1 and myosin-v defines Drosophila posterior determination. eLife 9, e54216 (2020).
Manseau, L. J. & Schüpbach, T. cappuccino and spire: two unique maternal-effect loci required for both the anteroposterior and dorsoventral patterns of the Drosophila embryo. Genes Dev. 3, 1437–1452 (1989).
Schonbaum, C. P., Perrino, J. J. & Mahowald, A. P. Regulation of the vitellogenin receptor during Drosophila melanogaster oogenesis. Mol. Biol. Cell 11, 511–521 (2000).
Greengard, L. & Rokhlin, V. A fast algorithm for particle simulations. J. Comput. Phys. 73, 325–348 (1987).
Stoddard, M. C. et al. Avian egg shape: form, function, and evolution. Science 356, 1249–1254 (2017).
Lu, W. et al. Ooplasmic flow cooperates with transport and anchorage in Drosophila oocyte posterior determination. J. Cell Biol. 217, 3497–3511 (2018).
Lu, W., Lakonishok, M. & Gelfand, V. I. Gatekeeper function for short stop at the ring canals of the Drosophila ovary. Curr. Biol. 31, 3207–3220.e4 (2021).
Spracklen, A. J., Fagan, T. N., Lovander, K. E. & Tootle, T. L. The pros and cons of common actin labeling tools for visualizing actin dynamics during Drosophila oogenesis. Dev. Biol. 393, 209–226 (2014).
Grieder, N. C., De Cuevas, M. & Spradling, A. C. The fusome organizes the microtubule network during oocyte differentiation in Drosophila. Development 127, 4253–4264 (2000).
Kass, M., Witkin, A. & Terzopoulos, D. Snakes: active contour models. Int. J. Comput. Vis. 1, 321–331 (1988).
Farhadifar, R. & Needleman, D. in Mitosis: Methods and Protocols (ed. Sharp, D.) Ch. 3 (Springer, 2014).
Zuiderveld, K. in Graphics Gems IV (ed. Heckbert, P. S.) 474–485 (1994).
Willert, C. E. & Gharib, M. Digital particle image velocimetry. Exp. Fluids 10, 181–193 (1991).
Thielicke, W. & Stamhuis, E. PIVlab—towards user-friendly, affordable and accurate digital particle image velocimetry in MATLAB. J. Open Res. Softw. 2, e30 (2014).
Jain, A. K. & Farrokhnia, F. Unsupervised texture segmentation using gabor filters. Pattern Recognit. 24, 1167–1186 (1991).
Acknowledgements
We thank B. Chakraborti, J.I. Alsous, E. Gavis and R. Goldstein for extensive and useful discussions and A. Farhadifar for generously sharing his Blender expertise. We acknowledge support from National Institutes of Health grant nos. R01GM134204 (S.Y.S.) and R35GM131752 (V.I.G.) and National Science Foundation grant no. DMR-2004469 (M.J.S.). Stocks obtained from the Bloomington Drosophila Stock Center, supported by National Institutes of Health grant no. P40OD018537, were used in this study. The computations in this work were performed at facilities supported by the Scientific Computing Core at the Flatiron Institute, a division of the Simons Foundation.
Author information
Authors and Affiliations
Contributions
M.J.S., S.Y.S. and V.I.G. designed the research. S.D., R.F., G.K. and R.B. contributed to simulation software development and simulation data analysis. W.L. and M.L. designed and performed the experiments. S.D., R.F. and M.J.S. developed the image processing software and analysis of experimental data. S.D., R.F., S.Y.S. and M.J.S. prepared the manuscript. All authors contributed to its editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Physics thanks Camille Duprat, Gerhard Gompper and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Notes 1 and 2 and Figs. 1–9.
Supplementary Video 1
Time course of configurations of microtubules anchored to the interior surface of a sphere in a cut-away view for a simulation with parameters \(\bar{\rho }=5\) and \(\bar{\sigma }=90\) (case I). The timestamp shows time normalized to the relaxation time of a single microtubule.
Supplementary Video 2
Time course of 2D projection of velocity field for a simulation with parameters \(\bar{\rho }=5\) and \(\bar{\sigma }=90\) (case I). The bordering circle represents the boundary of the sphere (case I). The timestamp shows time normalized to the relaxation time of a single microtubule.
Supplementary Video 3
Time course of configurations of microtubules anchored to the interior surface of a sphere in a cut-away view for a simulation with parameters \(\bar{\rho }=15\) and \(\bar{\sigma }=45\) (case II). The timestamp shows time normalized to the relaxation time of a single microtubule.
Supplementary Video 4
Time course of configurations of microtubules anchored to the interior surface of a sphere in a cut-away view for a simulation with parameters \(\bar{\rho }=15\) and \(\bar{\sigma }=45\) (case II). The timestamp shows time normalized to the relaxation time of a single microtubule.
Supplementary Video 5
Live video of a cross-section of a Drosophila oocyte obtained by a brightfield microscope. Scale bar, 50 μm. The timestamp is in units of minutes.
Supplementary Video 6
Time course of the polar order parameter P for a simulation with parameters \(\bar{\rho }=15\) and \(\bar{\sigma }=45\). Left and right views face two diametrically opposite defect-like structures formed in long time. The timestamp shows time normalized to the relaxation time of a single microtubule.
Supplementary Video 7
360∘ rotation of two snapshots at early time (t = 0.025τr, t = 0.035τr) and one snapshot at long time (t = 0.5τr) of a simulation with parameters \(\bar{\rho }=15\) and \(\bar{\sigma }=45\). τr is the relaxation time of a single microtubule. Surface polarity vectors pi corresponding to each microtubule are represented as arrows and superposed on the surface. The colour of the surface represents the polar order parameter P.
Supplementary Video 8
Time course of configurations of microtubules anchored to the interior surface for a cell shape similar to that of an oocyte, shown in two cut-away views. The simulation parameters are \(\bar{\rho }=15\) and \(\bar{\sigma }=45\). The timestamp shows time normalized to the relaxation time of a single microtubule.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dutta, S., Farhadifar, R., Lu, W. et al. Self-organized intracellular twisters. Nat. Phys. 20, 666–674 (2024). https://doi.org/10.1038/s41567-023-02372-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41567-023-02372-1