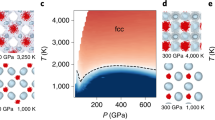
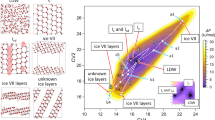
Abstract
Water confined in nanopores is ubiquitous in geological, planetary and biological environments and in nanofluidic settings. Understanding the phase behaviour and proton dynamics of nanoconfined water under high-pressure conditions is therefore important from both fundamental and applied points of view. Here we report a machine learning potential and present evidence from large-scale path-integral molecular dynamics simulations of the proton dynamics and phase behaviours of monolayer and bilayer ice under nanoconfinement and high pressures. We find that the symmetry breaking of the underlying hydrogen-bonding network of the two-dimensional (2D) ices together with strong nuclear quantum effects are responsible for the rich proton dynamics, such as the ultrafast one-dimensional proton-hopping within 2D ices. We also predict ten 2D ice phases. Notably, a 2D dynamic partially ionic phase and a superionic phase can be produced in the laboratory at pressures one order of magnitude lower than those measured for the bulk superionic phase or predicted for the partially ionic phase. We also identify a 2D solid-melting behaviour, namely consecutive double or triple continuous phase transitions from bilayer molecular ice to plastic ice and then to hexatic ice and the superionic fluid.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data, such as atomic structures and the machine learning force field, required to reproduce the key findings of this work, are available from the GitHub repository (https://github.com/jianjiang12/MLP-for-2D-ices). More detailed data are available from the corresponding authors upon request.
References
Loerting, T., Fuentes-Landete, V., Tonauer, C. M. & Gasser, T. M. Open questions on the structures of crystalline water ices. Commun. Chem. 3, 109 (2020).
Stanley, S. & Bloxham, J. Convective-region geometry as the cause of Uranus’ and Neptune’s unusual magnetic fields. Nature 428, 151–153 (2004).
Soderlund, K. & Stanley, S. The underexplored frontier of ice giant dynamos. Philos. Trans. R. Soc. A 378, 20190479 (2020).
Millot, M. et al. Experimental evidence for superionic water ice using shock compression. Nat. Phys. 14, 297–302 (2018).
Millot, M. et al. Nanosecond X-ray diffraction of shock-compressed superionic water ice. Nature 569, 251–255 (2019).
Prakapenka, V. B., Holtgrewe, N., Lobanov, S. S. & Goncharov, A. F. Structure and properties of two superionic ice phases. Nat. Phys. 17, 1233–1238 (2021).
Pickard, C. J., Martinez-Canales, M. & Needs, R. J. Decomposition and terapascal phases of water ice. Phys. Rev. Lett. 110, 245701 (2013).
Cavazzoni, C. et al. Superionic and metallic states of water and ammonia at giant planet conditions. Science 283, 44–46 (1999).
Liu, C. et al. Multiple superionic states in helium–water compounds. Nat. Phys. 15, 1065–1070 (2019).
Liu, C. et al. Plastic and superionic helium ammonia compounds under high pressure and high temperature. Phys. Rev. X 10, 021007 (2020).
Cheng, B., Bethkenhagen, M., Pickard, C. J. & Hamel, S. Phase behaviours of superionic water at planetary conditions. Nat. Phys. 17, 1228–1232 (2021).
French, M., Desjarlais, M. P. & Redmer, R. Ab initio calculation of thermodynamic potentials and entropies for superionic water. Phys. Rev. E 93, 022140 (2016).
Hernandez, J.-A. & Caracas, R. Superionic-superionic phase transitions in body-centered cubic H2O ice. Phys. Rev. Lett. 117, 135503 (2016).
Sun, J., Clark, B. K., Torquato, S. & Car, R. The phase diagram of high-pressure superionic ice. Nat. Commun. 6, 8156 (2015).
Wilson, H. F., Wong, M. L. & Militzer, B. Superionic to superionic phase change in water: consequences for the interiors of Uranus and Neptune. Phys. Rev. Lett. 110, 151102 (2013).
Hernandez, J.-A. & Caracas, R. Proton dynamics and the phase diagram of dense water ice. J. Chem. Phys. 148, 214501 (2018).
Benoit, M., Bernasconi, M., Focher, P. & Parrinello, M. New high-pressure phase of ice. Phys. Rev. Lett. 76, 2934 (1996).
Demontis, P., LeSar, R. & Klein, M. L. New high-pressure phases of ice. Phys. Rev. Lett. 60, 2284 (1988).
Wang, Y. et al. High pressure partially ionic phase of water ice. Nat. Commun. 2, 563 (2011).
Takii, Y., Koga, K. & Tanaka, H. A plastic phase of water from computer simulation. J. Chem. Phys. 128, 204501 (2008).
Zhao, W.-H. et al. Highly confined water: two-dimensional ice, amorphous ice, and clathrate hydrates. Acc. Chem. Res. 47, 2505–2513 (2014).
Zhu, C. et al. Computational prediction of novel ice phases: a perspective. J. Phys. Chem. Lett. 11, 7449–7461 (2020).
Algara-Siller, G. et al. Square ice in graphene nanocapillaries. Nature 519, 443 (2015).
Chen, J., Schusteritsch, G., Pickard, C. J., Salzmann, C. G. & Michaelides, A. Two dimensional ice from first principles: structures and phase transitions. Phys. Rev. Lett. 116, 025501 (2016).
Chen, J., Schusteritsch, G., Pickard, C. J., Salzmann, C. G. & Michaelides, A. Double-layer ice from first principles. Phys. Rev. B 95, 094121 (2017).
Jiang, J. et al. First-principles molecular dynamics simulations of the spontaneous freezing transition of 2D water in a nanoslit. J. Am. Chem. Soc. 143, 8177–8183 (2021).
Koga, K., Tanaka, H. & Zeng, X. C. First-order transition in confined water between high-density liquid and low-density amorphous phases. Nature 408, 564 (2000).
Ma, R. et al. Atomic imaging of the edge structure and growth of a two-dimensional hexagonal ice. Nature 577, 60–63 (2020).
Bai, J. & Zeng, X. C. Polymorphism and polyamorphism in bilayer water confined to slit nanopore under high pressure. Proc. Natl Acad. Sci. USA 109, 21240–21245 (2012).
Koga, K., Zeng, X. C. & Tanaka, H. Freezing of confined water: a bilayer ice phase in hydrophobic nanopores. Phys. Rev. Lett. 79, 5262 (1997).
Kapil, V. et al. The first-principles phase diagram of monolayer nanoconfined water. Nature 609, 512–516 (2022).
Lin, B., Jiang, J., Zeng, X. C. & Li, L. Temperature–pressure phase diagram of confined monolayer water/ice at first-principles accuracy with a machine-learning force field. Nat. Commun. 14, 4110 (2023).
Behler, J. & Parrinello, M. Generalized neural-network representation of high-dimensional potential-energy surfaces. Phys. Rev. Lett. 98, 146401 (2007).
Meier, T., Petitgirard, S., Khandarkhaeva, S. & Dubrovinsky, L. Observation of nuclear quantum effects and hydrogen bond symmetrisation in high pressure ice. Nat. Commun. 9, 2766 (2018).
Benoit, M., Marx, D. & Parrinello, M. Tunnelling and zero-point motion in high-pressure ice. Nature 392, 258–261 (1998).
Tian, Y. et al. Visualizing Eigen/Zundel cations and their interconversion in monolayer water on metal surfaces. Science 377, 315–319 (2022).
Ye, Q.-J., Zhuang, L. & Li, X.-Z. Dynamic nature of high-pressure ice VII. Phys. Rev. Lett. 126, 185501 (2021).
Kapil, V., Wilkins, D. M., Lan, J. & Ceriotti, M. Inexpensive modeling of quantum dynamics using path integral generalized Langevin equation thermostats. J. Chem. Phys. 152, 124104 (2020).
Rossi, M., Kapil, V. & Ceriotti, M. Fine tuning classical and quantum molecular dynamics using a generalized Langevin equation. J. Chem. Phys. 148, 102301 (2018).
Rossi, M., Liu, H., Paesani, F., Bowman, J. & Ceriotti, M. Communication: on the consistency of approximate quantum dynamics simulation methods for vibrational spectra in the condensed phase. J. Chem. Phys. 141, 181101 (2014).
Bronstein, Y., Depondt, P., Finocchi, F. & Saitta, A. M. Quantum-driven phase transition in ice described via an efficient Langevin approach. Phys. Rev. B 89, 214101 (2014).
Young, A. Melting and the vector Coulomb gas in two dimensions. Phys. Rev. B 19, 1855 (1979).
Kosterlitz, J. M. & Thouless, D. Long range order and metastability in two dimensional solids and superfluids. (Application of dislocation theory). J. Phys. C: Solid State Phys. 5, L124 (1972).
Singraber, A., Morawietz, T., Behler, J. R. & Dellago, C. Parallel multistream training of high-dimensional neural network potentials. J. Chem. Theory Comput. 15, 3075–3092 (2019).
Singraber, A. et al. CompPhysVienna/n2p2: Version 2.1.4. Zenodo. https://doi.org/10.5281/zenodo.4750573 (2021).
Hutter, J., Iannuzzi, M., Schiffmann, F. & VandeVondele, J. CP2K: atomistic simulations of condensed matter systems. Wiley Interdiscip. Rev. Comput. Mol. Sci. 4, 15–25 (2014).
Tribello, G. A., Bonomi, M., Branduardi, D., Camilloni, C. & Bussi, G. PLUMED 2: new feathers for an old bird. Comput. Phys. Commun. 185, 604–613 (2014).
Grimme, S., Ehrlich, S. & Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 32, 1456–1465 (2011).
Grimme, S., Antony, J., Ehrlich, S. & Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H–Pu. J. Chem. Phys. 132, 154104 (2010).
Lee, C., Yang, W. & Parr, R. G. Development of the Colle–Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 37, 785 (1988).
Becke, A. D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 38, 3098 (1988).
Lee, K., Murray, É. D., Kong, L., Lundqvist, B. I. & Langreth, D. C. Higher-accuracy van der Waals density functional. Phys. Rev. B 82, 081101 (2010).
Zhang, Y. & Yang, W. Comment on ‘Generalized gradient approximation made simple’. Phys. Rev. Lett. 80, 890 (1998).
Adamo, C. & Barone, V. Toward reliable density functional methods without adjustable parameters: the PBE0 model. J. Chem. Phys. 110, 6158–6170 (1999).
Brandenburg, J. G., Zen, A., Alfè, D. & Michaelides, A. Interaction between water and carbon nanostructures: how good are current density functional approximations? J. Chem. Phys. 151, 164702 (2019).
Pickard, C. J. & Needs, R. Ab initio random structure searching. J. Phys. Condens. Matter 23, 053201 (2011).
Ceriotti, M. & Manolopoulos, D. E. Efficient first-principles calculation of the quantum kinetic energy and momentum distribution of nuclei. Phys. Rev. Lett. 109, 100604 (2012).
Ceriotti, M., Bussi, G. & Parrinello, M. Nuclear quantum effects in solids using a colored-noise thermostat. Phys. Rev. Lett. 103, 030603 (2009).
Cheng, B. & Ceriotti, M. Computing the absolute Gibbs free energy in atomistic simulations: applications to defects in solids. Phys. Rev. B 97, 054102 (2018).
Ceriotti, M., More, J. & Manolopoulos, D. E. i-PI: A Python interface for ab initio path integral molecular dynamics simulations. Comput. Phys. Commun. 185, 1019–1026 (2014).
Thompson, A. P. et al. LAMMPS–a flexible simulation tool for particle-based materials modeling at the atomic, meso, and continuum scales. Comput. Phys. Commun. 271, 108171 (2022).
Singraber, A., Behler, J. R. & Dellago, C. Library-based LAMMPS implementation of high-dimensional neural network potentials. J. Chem. Theory Comput. 15, 1827–1840 (2019).
Humphrey, W., Dalke, A. & Schulten, K. VMD: visual molecular dynamics. J. Mol. Graph. 14, 33–38 (1996).
Larsen, A. H. et al. The atomic simulation environment—a Python library for working with atoms. J. Phys. Condens. Matter 29, 273002 (2017).
Hunter, J. D. Matplotlib: A 2D graphics environment. Comput. Sci. Eng. 9, 90–95 (2007).
Acknowledgements
C.Z. would like to acknowledge the National Key Research and Development Program of China (2021YFA 1500700) and the National Natural Science Foundation of China (Grant No. 22173011). X.C.Z. acknowledges computational support from the University of Nebraska Holland Computing Center and support from the Hong Kong Global STEM Professorship Scheme and the Research Grants Council of Hong Kong (GRF Grant No. 11204123).
Author information
Authors and Affiliations
Contributions
X.C.Z. and C.Z. conceived the project. J.J. performed the simulations. J.J., C.Z. and X.C.Z. analysed the data. J.J., C.Z., J.S.F. and X.C.Z. wrote the manuscript. All authors discussed the results and computational methods and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Physics thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–28.
Supplementary Video 1
Top and side views of ML-PL-Hexc in a 6-Å-wide nanopore at 440 K and 15.0 GPa through PIMD. The white and red balls represent the hydrogen and oxygen atoms, respectively.
Supplementary Video 2
Top and side views of ML-Hexc-SI in a 6-Å-wide nanopore at 1,000 K and 15.0 GPa through PIMD. The white and green balls represent the hydrogen atoms. The red balls represent the oxygen atoms.
Supplementary Video 3
Top and side views of BL-Sq-SI in a 6-Å-wide nanopore at 1,000 K and 45.0 GPa through PIMD. The white and green balls represent the hydrogen atoms. The red balls represent the oxygen atoms.
Supplementary Video 4
Top and side views of BL-iVII-SI in a 7-Å-wide nanopore at 80 K and 21.4 GPa through PIMD. The white and green balls represent the hydrogen atoms. The red balls represent the oxygen atoms.
Supplementary Video 5
Top and side views of BL-iVII-PL in a 8-Å-wide nanopore at 600 K and 11.2 GPa through PIMD. The white and red balls represent the hydrogen and oxygen atoms, respectively.
Supplementary Video 6
Top and side views of ZZMI in a 6-Å-wide nanopore at 80;K and 5.0 GPa through PIMD. The white and red balls represent the hydrogen and oxygen atoms, respectively.
Supplementary Video 7
Top and side views of bZZ-qBI in a 6-Å-wide nanopore at 80 K and 30.0 GPa through PIMD. The white and red balls represent the hydrogen and oxygen atoms, respectively.
Supplementary Video 8
Top and side views of ZZ-pMI in a 6-Å-wide nanopore at 80 K and 35.0 GPa through PIMD. The white and red balls represent the hydrogen and oxygen atoms, respectively.
Supplementary Video 9
Top and side views of ZZBI in a 6-Å-wide nanopore at 80 K and 45.0 GPa through PIMD. The white and red balls represent the hydrogen and oxygen atoms, respectively.
Supplementary Video 10
Top and side views of BL-iVII-FE in a 7-Å-wide nanopore at 80 K and 21.4 GPa through PIMD. The white and red balls represent the hydrogen and oxygen atoms, respectively.
Supplementary Video 11
Top and side view of BL-iVII-Zundel in a 7-Å-wide nanopore at 80 K and 64.3 GPa through PIMD. The white and red balls represent the hydrogen and oxygen atoms, respectively.
Supplementary Video 12
Top and side views of BL-iVII-FE' in an 8-Å-wide nanopore at 80 K and 18.8 GPa through PIMD. The white and red balls represent the hydrogen and oxygen atoms, respectively.
Supplementary Video 13
Top view of BL-Hexc-sSI in an 8-Å-wide nanopore at 1,000 K and 11.2 GPa through metadynamics simulations. The white (left panel) and green (left panel) balls represent the hydrogen atoms. The red balls (left panel) represent the oxygen atoms. The red (right panel) and blue (right panel) represent the oxygen atoms on the upper layer and lower layer, respectively.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jiang, J., Gao, Y., Li, L. et al. Rich proton dynamics and phase behaviours of nanoconfined ices. Nat. Phys. 20, 456–464 (2024). https://doi.org/10.1038/s41567-023-02341-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41567-023-02341-8