Abstract
Groups of animals can perform highly coordinated collective behaviours that confer benefits to the participating individuals by facilitating social information exchange and protection from predators1. Some of these characteristics could arise when groups operate at critical points between two structurally and functionally different states, leading to maximal responsiveness to external stimuli and effective propagation of information2,3. It has been proposed that animal groups constitute examples of self-organized systems at criticality2,3; however, direct empirical evidence of this hypothesis—in particular in the wild—is mostly absent. Here we show that highly conspicuous, repetitive and rhythmic collective dive cascades produced by many thousands of freshwater fish under high predation risk resemble a stochastic excitable system driven by environmental perturbations. Together with the results of an agent-based model of the system, this suggests that these fish shoals might operate at a critical point between a state of high individual diving activity and low overall diving activity. We show that the best fitting model, which is located at a critical point, allows information about external perturbations—such as predator attacks—to propagate most effectively through the shoal. Our results suggest that criticality might be a plausible principle of distributed information processing in large animal collectives.
Similar content being viewed by others
Main
Collective systems in biology—such as neuronal networks or large animal groups—are able to perform efficient collective information processing, although each unit or agent often only has access to local information1. It has been proposed that this feature of collective biological systems is due to systems operating at the boundary between dynamical regimes of different types, at critical points. At such critical points, the system’s macroscopic behaviour undergoes a qualitative change—that is, a phase transition4,2—and the system as a whole becomes highly sensitive to external perturbations, facilitating efficient collective information processing2,5,6. Critical or slightly sub-critical behaviour has been reported for neuronal systems5,7,8,9,10,11,12,13, but evidence for (near)-criticality in other collective information-processing systems, including animal groups, remains sparse2,4,14. In addition, studies of collective behaviour of animal groups under natural conditions beyond a few hundreds of individuals and over extended periods of time beyond a few seconds4,15 are missing. In this Letter we address this important gap by observing large groups of fish comprising hundreds of thousands of individuals16 over hours and in their natural ecosystem. By analysing this unique dataset, we provide evidence that giant fish shoals under high predation risk resemble a stochastic excitable system driven by environmental perturbations. By combining these empirical observations with computational modelling, we suggest that the fish system appears to operate close to criticality and that this could result in a near-optimal propagation of environmental cues, something that would confer benefits at the individual level in the corresponding ecosystem.
Our results are based on an investigation of the collective diving behaviour of sulphur molly (Poecilia sulphuraria) shoals. These fish are endemic to a sulfidic stream system near the city of Teapa in southern Mexico16,17 (Fig. 1a and Methods). Among several adaptations to toxic concentrations of hydrogen sulfide (H2S) as well as hypoxic conditions in their habitats17,18,19,20, these fish spend substantial parts of their time at the water surface, using the thin but oxygen-rich air–water interface to perform aquatic surface respiration21,22 (Supplementary Video 1). Typically, these fish aggregate in quasi-two-dimensional (2D) shoals of up to 3,000 individuals per square metre at the surface16, which suggests that the area covered in our recordings contained several hundred thousands of individuals. These surface-dwelling, high-density fish shoals attract various bird species as aerial predators at high frequencies16,22,23, and fish react to the attacking birds by exhibiting a highly synchronized collective diving behaviour. As the fish touch the water surface with their tails when initiating a dive, a characteristic and easily traceable water disturbance (visual splash—a wave) may spread through the shoal in an avalanche-like manner16,24 that resembles the ‘Mexican waves’ observed in football stadiums25. These large-scale repeated waves that appear after bird attacks function as a deterrent behaviour that leads to fewer and less successful attacks by birds16.
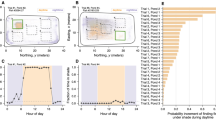
a, Localization of the field site in Teapa, Tabasco. The main pond, where all the videos were taken, is marked in the inset of the map. b, Snapshots of the rectification and background subtraction processes performed on the empirical videos. Active pixels caused by the disturbance of the surface as fish dive down are depicted in white. c, Plot of one surface-activity signal \({{{\mathcal{A}}}}{(t)}\) that is representative for all empirical videos. The inset serves to visualize the inter-spike time τ1 and the spike-duration time τ2. d,e, Plots of the distributions of the characteristic times P(τ1) (d) and P(τ2) (e) obtained from the empirical data. Both distributions are consistent with gamma distributions, which have exponentially decaying tails (KS statistic, p = 0.013 and p = 0.034, respectively), shown as black dashed lines. f, Plot of the distribution of the cluster areas P(a) obtained from the empirical data. The power-law fit (black dashed line) was estimated following ref. 30 (KS statistic, p = 0.044). For spatial scales ranging from 10−1 m2 up to 102 m2, the exponent is found to be αa ≈ 2.3. See Methods for details on the goodness-of-fit for all empirical distributions.
A key observation is that the synchronized collective diving behaviour, hereafter referred to as ‘surface-wave activity’, also occurs spontaneously in the absence of bird attacks, as seen in Supplementary Video 2. This spontaneous and stochastic surface-wave activity may be viewed as analogous to the resting-state activity in neuronal systems26,27. The system thus provides an optimal set-up for empirically testing the criticality hypothesis and investigating the benefits and potential trade-offs of critical behaviour in the wild28. Due to the high predatory bird activity in the system22,24, we hypothesized that these fish shoals would probably benefit from being in a constant state of alertness, operating at a critical point between two phases, one of low and another of high surface-wave activity. If so, the prediction would be to find intermittent surface-wave activity that can propagate through the whole system, giving rise to waves of different sizes ranging from small ones, involving a few individuals, to large ones involving most individuals in the shoals, which would follow a power-law distribution.
To test this hypothesis, we acquired videos, over multiple days, of the spontaneous and stochastic surface-wave activity in the absence of bird attacks, driven only by small-scale perturbations induced by external factors or uncertainty in individual perception (refs. 22 and 24 and Methods). Using a custom computer vision processing pipeline, we binarized the original videos into ‘active’ pixels that represent the diving of fish (state \({{{\mathcal{D}}}}\)) and ‘non-active’ pixels corresponding to fish during surface and underwater states \({{{\mathcal{S}}}}\) and \({{{\mathcal{U}}}}\) (see Fig. 1b and Methods for details). An example of the resulting processed videos is provided as Supplementary Video 3.
We first analysed the empirical surface-activity signal \({{{\mathcal{A}}}}{(t)}\), defined as the fraction of active pixels in the video, as a proxy for the number of fish diving at a given moment in time. We observed peaks of activity (‘spikes’), corresponding to waves spreading through the system, separated by long periods of low activity corresponding to small-scale, non-propagating surface activity (Fig. 1c, inset). Both the probability distribution of inter-spike time intervals τ1 and the probability distribution of spike-duration times τ2 have exponentially decaying tails (Fig. 1d,e and Extended Data Fig. 1). The mean time interval between spikes was significantly longer than their duration (〈τ1〉 = 12.84 ± 8.35 s and 〈τ2〉 = 0.68 ± 0.41 s, Wilcoxon one-sided signed-rank test, W = 1,523,502.5, p < 0.001), indicating that the propagation time of the waves is much smaller than the time between successive waves.
In a second step, we analysed how single waves spread through the shoals. To this end, we defined activity clusters as the number of active pixels connected in time and space corresponding to a single wave (as defined in ref. 29; see Methods for details). We found that the empirical cluster size distribution is consistent with a power-law distribution with an exponent of αa = 2.3 (Kolmogorov–Smirnov (KS) statistic30, p = 0.044; Fig. 1f and Extended Data Fig. 2). This exponent is robust across several decades, ranging from 10−1 m2 to 102 m2. The existence of a wave size distribution that is consistent with a power law suggests that the system operates at criticality12.
However, a power-law distribution in empirical data is not sufficient, alone, to conclude that a system is at criticality12. Thus, to provide further evidence that the observed surface-wave activity is at the edge between two different dynamical regimes, we devised a generic model for the spatiotemporal propagation of the surface-activity waves. In this way we can simulate the emergent collective dynamics and identify parameters best fitting the observed macroscopic behaviour. Our prediction here is that if the fish shoals are operating at a critical point, we will find that the parameters that best fit the data are indeed located at a phase transition (critical point). One way to quantify this is to calculate the average correlation of fluctuations between neighbouring cells, which peaks at the phase transition6,12.
We modelled the spontaneous surface-wave activity in a similar manner to Farkas and others25. The surface is subdivided into spatial cells, each representing the mean dynamics of a subset of fish. Each cell can be in one of three states, corresponding to the stereotypical behaviours \({{{\mathcal{S}}}}\) (surface), \({{{\mathcal{D}}}}\) (dive) or \({{{\mathcal{U}}}}\) (underwater), as seen in the scheme in Fig. 2a. Cells enter the diving state stochastically, either spontaneously by environmental noise or driven by neighbouring cells31, making the propagation of diving events possible. The model has five free parameters: two time constants that control how long the cells remain in the diving and underwater state, two parameters that control the stochasticity of spontaneous events (parameters ω and μ) and—crucially—a coupling parameter θ, which represents an activation threshold and controls how many neighbouring cells need to be activated to prompt a given cell to become active. We first matched the time constants to previously published experimental data16 before performing a systematic parameter search to identify the values ω⋆, θ⋆ and μ⋆ that best fit the surface-wave activity of the empirical system (Extended Data Fig. 3). The fit optimized two quantities, one quantifying the temporal dynamics of the system and one characterizing the local spatial dynamics (see Methods and Extended Data Figs. 4 and 5 for details). The best fitting model reproduces the empirical data, both qualitatively (snapshots of the dynamics and surface-activity signal are shown in Fig. 2b,c, respectively) and quantitatively in terms of the distributions of the characteristic times P(τ1) and P(τ2) (Fig. 2d,e), as well as the cluster size distribution P(a) (Fig. 2f). We note that in the analysis of the simulation results we used time series of the same length as were available from experiments (Methods). Thus, the exponential tails in the inter-spike distributions are probably induced by the relatively short observation windows due to experimental constraints.
a, We categorized the behaviour of individual fish into three fundamental states: swimming near the surface for respiration (\({{{\mathcal{S}}}}\)), fast diving (\({{{\mathcal{D}}}}\)) and underwater hovering with subsequent slow resurfacing (\({{{\mathcal{U}}}}\))24. b, Comparison between snapshots obtained from the empirical videos and the numerical simulations. c, Surface-activity signal \({{{\mathcal{A}}}}{(t)}\) computed using the model shown in a. d,e, Characteristic time distributions for the inter-spike times τ1 (d) and the spike-duration times τ2 (e). f, Distribution of cluster areas P(a), which is statistically consistent with a power law (black dashed line) with exponent αa = 2.3. g, Plots of the surface-activity signal \({{{\mathcal{A}}}}{(t)}\) for three different sets of parameters, highlighted with different markers in h. h, Average neighbour correlation function \({\langle c(\omega ,\,\theta ,\,{\mu }^{\star })\rangle }_{t}\) showing a maximum at the critical region. The blue cross indicates the location of (ω⋆, θ⋆). i, Plots of \({\langle c({\omega }^{\star },\,\theta ,\,{\mu }^{\star })\rangle }_{t}\) and \({\langle c(\omega ,\,{\theta }^{\star },\,{\mu }^{\star })\rangle }_{t}\) to visualize the maximum when changing variables θ and ω. The plots in b–f were generated using numerical simulations and the parameters ω⋆, θ⋆ and μ⋆. For details, see the section Model implementation in Methods.
By varying the spontaneous and coupling parameters ω and θ while fixing the other parameters to their best fitting values, we find that the ones that best fit the data are indeed located at a phase transition (critical point), where the system behaviour undergoes a fundamental change mainly with respect to variations in the coupling parameter θ. For stronger coupling (lower θ), the system exhibits high levels of persistent activity, effectively spanning the whole system. For weaker coupling (higher θ), we observe only small non-propagating, purely noise-driven activations (Fig. 2g and Supplementary Video 4). As predicted, the model shows a maximal average correlation at a specific value of the coupling parameter θ and remains largely unaffected by the spontaneous transition rate ω, as seen in Fig. 2h–i (see Methods for details). This means that variation of the social interactions among individuals is responsible for moving the system across the activity phases. To further support that the observed transition in the computational model is indeed a critical one, we computed the susceptibility of the surface-activity signal \({{{\mathcal{A}}}}{(t)}\) as a function of system size (L ∈ [25, 50, 100, 200, 400, 800]). We found that the susceptibility increases as a function of system size in a way that is consistent with a power law with exponent ≈ 1.7 (Extended Data Fig. 6). As a complement to this numerical analysis of the critical transition in the model, we computed the susceptibility in the empirical videos using different window sizes. We found that, in the empirical data, the susceptibility of the activity also increases with increasing window size (Extended Data Fig. 7). The exponents vary across measurement days—potentially due to environmental factors such as temperature, or lighting conditions—but they are consistent with the exponent in the model for at least three measurement days. Thus, the combination of these numerical and empirical results suggests that the system operates in close vicinity to a critical point. Details on the analysis of the critical transition are provided in Methods (see section Analysis of the critical transition).
To investigate the possibility that the individual fish in shoals at the critical point could benefit from optimal sensitivity to environmental stimuli32, as well as efficient information propagation across large distances33, we combined two different computational approaches. First, using the numerical model, we tested the sensitivity of the system when exposed to external perturbations of different intensities, analogous to ref. 34. At random points in time we activated a number \({{{\mathcal{I}}}}\) of cells in a perturbation zone (PZ in Fig. 3a) always located in the centre of the system, and observed its response in a sampling zone (SZ in Fig. 3a), which consisted of a larger set of cells excluding the cells of the PZ. The sizes of the PZ and SZ were selected such that the size of the SZ is one order of magnitude larger than the PZ. We characterized the ability of the system to detect a perturbation of a given intensity \({{{\mathcal{I}}}}\) by calculating the surface-activity signal of the cells in the SZ (\({{{{\mathcal{A}}}}}_{\rm{SZ}}{(t)}\)) and then computing its time average. We then implemented receiver operating characteristic (ROC) analysis to quantify the effect of the perturbations on the system. To obtain a binary classification, we implemented numerical simulations with no perturbation (true negative (TN) case) and compared them with simulations perturbed with the five different intensities (true positive (TP) cases). For each perturbation intensity \({{{\mathcal{I}}}}\), we computed the area under the curve (AUC) of the false positive (FP) versus TP curve. We found that the average sensitivity ΦAUC(ω, θ, μ⋆) is maximal at the critical boundary, as seen in Fig. 3b (see Methods and Extended Data Fig. 8 for details). Again, the parameters that best fit the empirical observations (highlighted with a blue cross in Fig. 3b) are close to this boundary, suggesting that the experimentally observed shoals could maximize their sensitivity to external perturbations.
a, Illustration of the PZ and SZ for sensitivity analysis. b, Heatmap showing the values of the parameter ΦAUC(ω, θ, μ⋆). The blue cross denotes the location of the parameters (ω⋆, θ⋆). c, Scheme to describe the training of the RNN for different parameters (ω, θ) to classify the event of a perturbation. The training was implemented using the BPTT technique (Methods). d, Scheme to visualize the PZ and SZ for analysis of the propagation of information through the system. e, Heatmap showing the accuracy of the RNN. We find that the empirically fitted parameters (ω⋆, θ⋆) (highlighted with a blue cross) are located where the accuracy peaks.
Second, we characterized the propagation of information through the system by classifying the presence of a perturbation based on the activity in a small SZ at varying location (Fig. 3d). This localized subset of cells aims to emulate the local perspective of small groups of fish in the system. We used a generic machine-learning-based classifier to probe the general ability of the system to identify the perturbations to the system. For this, we used the time-dependent stochastic activation patterns in the SZ from the simulated data. We computed the results in the parameter space defined by θ and ω, in particular the performance at the set of parameters that best fit the experimental observations. We used a recurrent neural network (RNN; LSTM, long short-term memory)35 to integrate the local activity information over time (Fig. 3c, Methods and Extended Data Table 1). For each parameter pair (θ, ω), we trained a separate network, providing a binary prediction at every time step (Fig. 3c). This result, shown in Fig. 3e, is qualitatively similar to the sensitivity analysis (Fig. 3b), suggesting that individuals can detect the presence of a perturbation at a given distance optimally only when the system operates close to the critical region. Taken together, the above analysis demonstrates that, in the model, the critical state facilitates precise and robust propagation of information through the system, something that might also be present in the real-life system.
In summary, by combining empirical data with mathematical modelling, we have shown that the spatiotemporal collective dynamics of large shoals of sulphur mollies correspond to a noise-driven excitable system at criticality. Our estimated exponent for the cluster size distribution is consistent with those observed for the closely related self-organized critical forest fire model by Drossel and Schwabl36. However, as noted by Grassberger37, corresponding estimations are difficult due to strong finite size effects. We further refrain from claims on the exponent belonging to a particular universality class. Although this is a fundamental concept in equilibrium statistical physics, the general relevance of universality for non-equilibrium phase transitions is under debate due to reports of non-universal, parameter-dependent scaling exponents38. A corresponding in-depth investigation of the scaling exponents and scaling relationship is beyond the scope of this work. Furthermore, using two different computational classifying methods, we have shown that operating at the critical point maximizes the discrimination ability of (local) environmental cues of different intensities, as well as the communication range between individuals in different locations of the system. In such natural systems, cue intensity scales with danger, as hunting birds often enter the water with large parts of their bodies, leading to high-intensity visual, acoustic and hydrodynamic perturbations, while overflying birds barely provide visual cues24. Thus, information on the cue intensity is highly relevant for the fish in order to coordinate appropriate responses, including repeated diving for multiple minutes16. In addition, this information can be communicated across wide distances, allowing fish to take action even when not within the direct area of danger. Being at a critical point potentially allows the sulphur molly shoals to be in a constant state of alertness to environmental perturbations and information on the cue intensity that relates to the danger of these perturbations, which can then be passed most effectively through the shoals. This would suggest that there could be adaptive benefits of the critical state in terms of optimal information processing39. This wide range of empirical observations points to (self-organized) criticality as a possible general organizing principle of collective information processing2. Nevertheless, there are also important differences between our system and the other biological systems mentioned above. For example, in neuronal systems, the structure of the interaction network between individual elements changes on a much slower timescale than the dynamical behaviour of the network, so the networks may be assumed to be effectively constant. Such timescale separation is assumed essential for self-tuning of these systems to criticality10,40. For example, in starling flocks, signatures of criticality have been reported based on short-term observations of highly ordered flocks33, where network rearrangements are negligible2,41. For the sulphur molly shoals, the interaction networks between individuals are highly dynamic and change on timescales comparable to the observed behaviour. Thus, our results suggest that self-organized critical behaviour could be a robust feature of biological information processing not requiring such timescale separation, while at the same time pose new fundamental questions on the theoretical description and underlying mechanisms of self-organized adaptation towards criticality.
It remains to be studied how the sulphur mollies—and similar systems that show characteristics of criticality—mechanistically tune themselves towards critical points. Ultimately, criticality has to emerge from the adaptation of individual-level behaviour, such as effective social interactions3,39,42. It has been shown that the density of sulphur molly shoals exhibits substantial variation over the course of a day22, which in turn modulates the effective strength of social interactions31,43. This suggests local density to be one core variable controlling the self-organization of living systems to criticality28 and thus a variable to quantify over longer periods of time. However, individual behavioural parameters such as individual speed44 or attention to conspecifics45 have been shown to have strong effects on collective behaviour, which may yield alternative mechanisms for self-organization towards criticality through modulation of individual behaviour46. More generally, this relates also to the recently raised broader question on whether—and if so how—natural collectives and bioinspired artificial systems are capable of flexibly controlling their distance to criticality to modulate their information-processing capabilities depending on the environmental context28,46.
Methods
Collection of field observations
The field observations were performed in Teapa, a municipality in Tabasco in south-eastern Mexico (17° 33′ N, 93° 00′ W). In this area, there are several sulfidic springs that feed into surrounding surface–water streams and pools. The field work and data collection were performed from 6 April to 11 April 2018 in the largest pond (~600 m2) in the Baños del Azufre spring complex (Fig. 1a). A total of 89 videos were collected, each between 120 and 180 s long. This relatively short length of video recordings was chosen to ensure that, during the observation, as well as at least 180 s before the time window, the system had not been subject to a large external perturbation like a bird attack or bird flyover, to obtain the best possible approximation of spontaneous fish activity given the experimental constraints. The videos were recorded in the afternoon (17:00 to 19:00). The frame rate acquisition of all videos was 50 frames s−1. Figure 1b provides a snapshot.
Rectification process
Each of the videos was rectified to reconstruct a top view of the pond. For this purpose, we positioned a 1.55 m × 1.55 m plastic square on the surface of the water at the end of each recording session. We rectified the videos using the four corner positions of the square and the OpenCV Python library47. The rectification process is shown in Fig. 1b.
Background subtraction process
We implemented the background subtraction process to each rectified video using the MOG2 background subtractor from the OpenCV library in Python48. After the background subtraction process, the obtained videos consisted only of black and white pixels, as seen in Fig. 1b. For this purpose we used a threshold value of 100 and a history value of 400 in the OpenCV function createBackgroundSubtractorMOG248. We cropped the processed videos to eliminate the black edges of the videos created during the rectification process, as well as the parts of the pond close to the shore, thus keeping only the dynamics in the centre of the pond. The size of all the processed empirical videos is 500 × 500 pixels.
Analysis of the surface-activity signal
We quantified the surface-wave activity of the pond using the processed black-and-white videos. We computed the surface-activity signal \({{{\mathcal{A}}}}{(t)}\), which is defined for each time step (or frame) as the number of white pixels at a given time t divided by the total number of pixels in that frame. Thus, the surface-activity signal \({{{\mathcal{A}}}}{(t)}\) can take values between 0 and 1. From each empirical signal we calculated two quantities that served to characterize the dynamics. These quantities are the mean value \({\langle {{{\mathcal{A}}}}\rangle }_{t}\) and the threshold value \({{{{\mathcal{A}}}}}_{{{{\rm{th}}}}}\). They are defined as
where T is the time duration of the signal. We computed a third quantity from each signal, which is the ratio of both previously defined values:
We used this ratio \({{{\mathcal{R}}}}\) to compare the empirical signals of \({{{\mathcal{A}}}}{(t)}\) with the corresponding signals obtained in numerical simulations. The average value over all empirical videos was \({{{{\mathcal{R}}}}}_{{{{\rm{emp}}}}}={0.33\pm 0.15}\). The numerical values of these quantities are presented in Extended Data Fig. 4 for each video. We used the threshold value \({{{{\mathcal{A}}}}}_{{{{\rm{th}}}}}\) to compute the characteristic times τ1 (inter-spike time) and τ2 (spike-duration time), shown in Fig. 1c. The corresponding distributions obtained from the empirical videos are presented in Fig. 1d,e.
Fitting of characteristic times distributions and goodness-of-fit
We fitted the empirically obtained distributions for the characteristic times τ1 and τ2 using a gamma distribution:
where κ > 0 and Ω > 0 are two free parameters of the distribution and Γ(κ) is the gamma function. We can fit the two parameters of equation (4) using the first two moments of the empirical distributions by computing
where σ2 = 〈(τ − 〈τ〉)2〉. In this way, we obtain the best candidate to fit the empirical data. To assess the goodness of this fit, we followed a procedure introduced in ref. 30. We first generate a set of random data sampled using a gamma distribution with the parameters κ and θ fitted from the empirical data. We then calculate the D-statistics using the KS distance:
where Fsynth(τ) in equation (6) is the cumulative distribution function (CDF) of the synthetically generated data, and Ftheory(τ) is the analytic expression of the CDF of the gamma distribution in equation (4), which is
where γ(x, y) is the lower incomplete gamma function, defined as
We repeat this process 2,500 times and generate the distribution P(D) with all the obtained values. We now calculate the value of the D-statistics using the empirical data by computing
where Femp(τ) is the CDF of the empirical data. We compare the numerical value of Demp with the distribution generated with the synthetic data and compute the p value as the fraction of values of D lower than Demp. We show the results in Extended Data Fig. 1 for both characteristic times τ1 and τ2. The low p values obtained for both characteristic times (p = 0.013 and p = 0.034, respectively) suggest that the error between the empirical measurements and the analytic expression in equation (4) can be associated only to noise30. We can thus conclude that their distributions are consistent with gamma distributions, which have exponentially decaying tails.
Computation of activity clusters
The activity cluster analysis presented in the main text was implemented using the definition given in ref. 29. We quantified the activity clusters (coherent spatiotemporal structures) by stacking the images of each black-and-white video at equal time intervals Δt and then counting the number of connected spatiotemporal white pixels. The cluster volumes are then defined as the size of these spatiotemporal structures. If we collapse the cluster volumes over the temporal axis, we obtain purely spatial structures that we call cluster areas. The distributions of the cluster volumes and cluster areas are shown in Extended Data Fig. 2. We also computed the speed of each cluster to filter small-sized ones, related to moving objects on the surface of the pond. We used the same methods as used in ref. 16, and we analysed only the clusters with a speed higher than 0.5 m s−1 to be sure that the analysed clusters were originated by fish activity.
Power-law analysis of the cluster distributions
We implemented an analysis on the empirical distributions of the cluster areas and volumes to quantify how far these distributions are statistically consistent with a power-law distribution of the form
where v and a stand for the cluster volumes and cluster areas, respectively. We performed the analysis following ref. 30 and using the Python power-law library49. There are two important values that need to be calculated from the data: the minimal value \({a}_{\min }\) (and \({v}_{\min }\)) and the exponent αa (and αv). With these minimal values \({a}_{\min }\) and \({v}_{\min }\), we can calculate the exponents using maximum likelihood estimators30:
where ai (with i = 1, …, na) and vi (with i = 1, …, nv) are the observed empirical values such that \({a}_{i} > {a}_{\min }\) and \({v}_{i} > {v}_{\min }\). Following ref. 30, we obtain the best fitting parameters \({a}_{\min }^{\star }\), \({v}_{\min }^{\star }\), \({\alpha }_{a}^{\star }\) and \({\alpha }_{v}^{\star }\) and we compute the corresponding D-statistics for areas and volumes, which we call \({D}_{a}^{\star }\) and \({D}_{v}^{\star }\). To test the goodness of the fit of these parameters, we generate random data sampled from a power-law distribution with the estimated parameters, analogous to what we explained in the previous section. We then obtain the distributions P(Da) and P(Dv) and compute the corresponding p values as the fraction of the synthetically generated data where \({D}_{a}\le {D}_{a}^{\star }\) and \({D}_{v}\le {D}_{v}^{\star }\), respectively.
Model implementation
The system consists of a square lattice of L × L cells. The temporal dynamics of the cells is stochastic, and it is computed with a discrete variable that we call q(i, j, t), where i and j are integers that represent the location of the cell within the system, and t is the time. Each cell can be in one of three different states (\({{{\mathcal{S}}}},\,{{{\mathcal{D}}}},\,{{{\mathcal{U}}}}\)), each one associated to one behaviour as explained in the main text. A snapshot of the numerical simulations is shown in Fig. 2b and in Extended Data Fig. 3a. The transition rate that controls the change of cell ij from state \({{{\mathcal{S}}}}\) to state \({{{\mathcal{D}}}}\) is mathematically expressed as
where x is the number of neighbours in state \({{{\mathcal{D}}}}\) of the cell ij. The neighbourhood of each cell is defined as its eight nearest neighbours (Moore neighbourhood; Extended Data Fig. 3b,c). For the interaction term in equation (14) we use a sigmoid function with two parameters called θ and μ:
Examples of equation (15) are provided in Extended Data Fig. 3d,e. The spontaneous term in equation (14) is given by
with ω a constant. Notice that the probability of observing a spontaneous transition \({{{\mathcal{S}}}}\to {{{\mathcal{D}}}}\) in the complete system increases with increasing system size as L2. We are then left with a total of three free parameters in the model: ω, θ and μ. The transitions \({{{\mathcal{D}}}}\to {{{\mathcal{U}}}}\) and \({{{\mathcal{U}}}}\to {{{\mathcal{S}}}}\) were implemented in a deterministic way. This means that a cell that transitioned to state \({{{\mathcal{D}}}}\) would remain in this state (and thus affect its neighbours that are in state \({{{\mathcal{S}}}}\)) for a time \({t}_{{{{\mathcal{D}}}}}\). After this time, the cell would change its state to \({{{\mathcal{U}}}}\). Analogously, a cell in state \({{{\mathcal{U}}}}\) remains a time \({t}_{{{{\mathcal{U}}}}}\) in this state before changing back to state \({{{\mathcal{S}}}}\). These transitions can also be implemented in a stochastic fashion using constant rates such that \({R}_{{{{\mathcal{D}}}}}={1}/{t}_{{{{\mathcal{D}}}}}\) and \({R}_{{{{\mathcal{U}}}}}={1}/{t}_{{{{\mathcal{U}}}}}\). Notice that the stochastic implementation of the transitions \({{{\mathcal{D}}}}\to {{{\mathcal{U}}}}\) and \({{{\mathcal{U}}}}\to {{{\mathcal{S}}}}\) requires the use of a total of three states (\({{{\mathcal{S}}}}\), \({{{\mathcal{D}}}}\), \({{{\mathcal{U}}}}\)) to have a refractory period between two succesive events where a single cell was in the susceptible state \({{{\mathcal{S}}}}\) (ref. 50). For our simulations, we used the values of \({t}_{{{{\mathcal{D}}}}}={1}\) s and \({t}_{{{{\mathcal{U}}}}}={3}\) s. These values correspond to the observations published in ref. 24, where experiments were performed in a controlled environment in the laboratory using small groups of sulphur mollies that were captured in the same sulfidic springs in Teapa. In their experiments, the fish were exposed to artificial visual and acoustic stimuli presented separately or combined. They measured—among other observables—the diving duration (called fast-start duration in Fig. 4c of ref. 24), as well as the total dive duration (shown in Fig. 4e of ref. 24). The numerical values of \({t}_{{{{\mathcal{D}}}}}\) and \({t}_{{{{\mathcal{U}}}}}\) used in our simulations were chosen to reproduce the observations done for the bimodal stimulus.
For the numerical results presented in Fig. 2 in the main text, we used the Euler–Maruyama method51 to compute the time evolution of the model. For all panels in the figure, the time step was Δt = 0.1s and the system size was L × L = 250,000 cells. For Fig. 2c,g, the number of time steps was 1,300 (equal to 130s) and only one realization was required for each plot. For Fig. 2d–f, the number of time steps was 5,000,000 and, again, only one realization was required to acquire the data for the three distributions. Finally, for Fig. 2h,i, the number of time steps was 50,000 (per realization) and 10,000 realizations were computed for each set of parameters (neighbour coupling θ and spontaneous rate ω). The value of each point in Fig. 2h,i is the average correlation function over all realizations. For details on the computation of the results in Fig. 2h,i, see the Average correlation function section.
Fitting of the parameters of the model
We fitted the parameters ω, θ and μ by comparing the empirical data with the numerical simulations via two quantities, the first being the previously defined ratio \({{{\mathcal{R}}}}\) and the second one the average number of active neighbouring cells, which we call \({{{\mathcal{N}}}}\). The ratio \({{{\mathcal{R}}}}\) is a quantity related to the temporal dynamics of the system, and \({{{\mathcal{N}}}}\) is instead related to the spatial properties of the observed patterns in the natural system. We computed \({{{\mathcal{N}}}}\) by randomly selecting time frames from each video and computing the number of neighbouring cells in state \({{{\mathcal{D}}}}\) of randomly selected focal cells, as depicted in Extended Data Fig. 5. From each video we sampled 2,000 randomly selected frames, and we selected 2,500 cells from each frame as focal cells. The average value obtained over all empirical videos is \({{{{\mathcal{N}}}}}_{{{{\rm{emp}}}}}={0.27\pm 0.23}\). To find the best fitting parameters, we define two auxiliary functions, \({\epsilon }_{1}{(\omega ,\,\theta ,\,\mu )}=| {{{{\mathcal{R}}}}}_{{{{\rm{emp}}}}}-{{{{\mathcal{R}}}}}_{{{{\rm{sim}}}}}{(\omega ,\,\theta ,\,\mu )}|\) and \({\epsilon }_{2}{(\omega ,\,\theta ,\,\mu )}=| {{{{\mathcal{N}}}}}_{{{{\rm{emp}}}}}-{{{{\mathcal{N}}}}}_{{{{\rm{sim}}}}}{(\omega ,\,\theta ,\,\mu )}|\), where \({{{{\mathcal{R}}}}}_{{{{\rm{sim}}}}}{(\omega ,\,\theta ,\,\mu )}\) and \({{{{\mathcal{N}}}}}_{{{{\rm{sim}}}}}{(\omega ,\,\theta ,\,\mu )}\) are the resulting values obtained in the numerical simulations for a given set of parameters (ω, θ, μ). For a fixed value of μ, we can plot the two functions ϵ1 and ϵ2 in the 2D space defined by ω and θ. The corresponding plots for the case μ = 1 are shown in Extended Data Fig. 3f,g. When we compute the values of ω and θ that minimize the functions ϵ1 and ϵ2 (Extended Data Fig. 3f,g, orange lines), we can find the set of parameters ω⋆ and θ⋆ that minimize both auxiliary functions simultaneously, namely the crossing of both orange lines, highlighted with a blue cross in Extended Data Fig. 3f,g. We can look for the corresponding parameters ω⋆ and θ⋆ for every given value μ. We implemented numerical simulations for different values of μ and compared them via the function \({{{\widehat{\boldsymbol{\epsilon }}}}}{({\omega }^{\star },\,{\theta }^{\star },\,\mu )}={\epsilon }_{1}{({\omega }^{\star },\,{\theta }^{\star },\,\mu )}+{{\epsilon }_{2}({\omega }^{\star },\,{\theta }^{\star },\,\mu )}\). The values of \({{{\widehat{\boldsymbol{\epsilon }}}}}{({\omega }^{\star },\,{\theta }^{\star },\,\mu )}\) are shown in Extended Data Fig. 3h. Notice that the minimal value is reached for a value of μ = μ⋆ = 1. Thus, the best fitting parameters are ω⋆ = 0.6 × 10−5, θ⋆ = 2.5 and μ⋆ = 1.
Analysis of the critical transition in the model
To provide further evidence that the transition observed in the model is indeed critical, we analysed the susceptibility of the mode as a function of the system size. For this, we highlight that the phase transition is mainly driven by the neighbour coupling θ, as observed in Fig. 2h, Fig. 3b,e and Extended Data Fig. 3g. Thus, to simplify the analysis, we fixed the spontaneous rate ω and parameter μ to their optimal values (ω⋆, μ⋆) and performed a more thorough analysis by varying only the parameter θ. For a given value of this parameter, we computed the average mean square fluctuations of the activity signal \({{{\mathcal{A}}}}{(t)}\)—that is, the susceptibility—defined as
where L is the system size (and L × L is the number of cells in the system). We computed the susceptibility χ for six different system sizes: L ∈ [25, 50, 100, 200, 400, 800]. We show the results in Extended Data Fig. 6a. In all cases, and for all system sizes, the susceptibility peaks in an interval between θ = 2 and θ = 3 (approximately). We can easily notice that the numerical value of the susceptibility at the peak increases with increasing system size L. We obtained the value of θ at which the susceptibility χ reaches its maximum value (peak) and called it θc. We compared the effect of the system size L on the values of the susceptibility for three different regimes: θ < θc, θ = θc and θ > θc. The results are shown in Extended Data Fig. 6b, where we can see that the numerical value of the susceptibility increases with increasing system size L only for the case θ = θc, and remains constant for the other two regimes. In that case, susceptibility χ diverges with an exponent of ~1.7.
To better quantify this result, we computed the width of the peak of the susceptibility χ—called \({{{\mathcal{W}}}}\)—defined as
where N is the sum of susceptibility values \({\mathop{\sum }\nolimits_{i = 1}^{N}{\chi }_{i}}\), θc is the value for which the susceptibility reaches its maximum for each system size L, and χi and θi are the numerical values plotted in Extended Data Fig. 6a for all system sizes. The results are plotted in Extended Data Fig. 6c for all system sizes, where we observe that the width \({{{\mathcal{W}}}}\) decreases with increasing system size L. From these results we can conclude that the transition we observe in the model is indeed of a critical nature, given that the susceptibility peaks at θc (a good method to identify the critical regime), and the larger the system size L, the larger and thinner the peak.
Computation of the susceptibility in empirical data
As a complement to the analysis of the critical transition in the model, we computed the susceptibility—as defined in equation (17)—in the empirical videos. For this, we used different window sizes LW of the original videos and computed the surface-activity signal \({{{\mathcal{A}}}}{(t)}\) in that window. We show the results in Extended Data Fig. 7, where we notice that, in all cases, the susceptibility increases with window size, which strongly suggests that the real-life system operates at criticality. We highlight that the the best fitting power laws for the different acquisition days have an exponent between 1 and 2. As shown in the previous subsection, the exponent obtained in the model (numerical value of ~1.7) lies within the same range. Furthermore, there are three days (180407, 180410 and 180411) where the best fit to the empirical data is indeed very close to the numerical result of the model. There are different potential explanations for this variability observed in the exponent: the different camera set-ups or camera position or the time of the day at which the videos were taken. Furthermore, the distribution of the fish in the region of interest may vary slightly over the course of different recordings, which, together with finite size effects, may lead to variation in the corresponding exponents. A corresponding detailed investigation is beyond the scope of this work.
Average correlation function
To quantify the correlation of the fluctuations of the activity of the system, we computed the average correlation function 〈c(ω, θ, μ)〉, as done in ref. 15. We first computed the time average value of the variable q(i, j, t) by calculating
where T is the observation time and q(i, j, t) = 1 if the cell is in the active state \({{{\mathcal{D}}}}\) and q(i, j, t) = 0 otherwise. We can then compute the correlation of the fluctuations of all cells in the system with its neighbours at a given time t:
where Ωij is the set of neighbours of a given cell ij. The resulting function in equation (20) depends on the values of the parameters ω, θ and μ. To compare the correlation for different parameter values, we computed the time average of equation (20) as
For the plot in Fig. 2h we computed the average value of \({\langle c(\omega ,\,\theta ,\,{\mu }^{\star })\rangle }_{t}\) over 10,000 realizations, where each realization consisted of 50,000 time steps. Cuts at ω = ω⋆ and θ = θ⋆ are also shown in Fig. 2i.
Perturbation analysis
We explored the effect of perturbations on the numerical simulations of the model. For this purpose, we considered five different perturbation intensities by perturbing a different number of cells in the PZ (Fig. 3a). The total area of the PZ—called \({{{{\mathcal{V}}}}}_{\rm{PZ}}\)—represents 1.5% of the total area of the system. The main reason is that we aim to study the effect of strong and highly localized perturbations on the system. For the results presented in the main text, we used a system of size L × L = 25 × 25 cells (this is, a localized analysis) and thus the total area of the PZ was \({{{{\mathcal{V}}}}}_{\rm{PZ}}={3\times 3}\) cells. The five different intensities were selected to represent a fraction of \({{{{\mathcal{V}}}}}_{\rm{PZ}}\) such that \({{{\mathcal{I}}}}\in {\{1,\,3,\,5,\,7,\,9\}}\).
Sensitivity analysis
We implemented the ROC analysis to quantify the effect of the perturbations on the system. To obtain a binary classification, we implemented numerical simulations with no perturbation (TN case) and compared them with simulations perturbed with the five different intensities (TP cases). For all cases, we sampled the dynamics of all cells in the sample zone SZ (Fig. 3a) of total area \({{{{\mathcal{V}}}}}_{\rm{SZ}}={3\times 3}\) cells. We computed the activity signal of the cells in the SZ—called \({{{{\mathcal{A}}}}}_{{{{{\rm{SZ}}}}}}{(t)}\)—for an observation time T = 2,000, and we computed the time average:
We implemented 5,000 realizations (using the Euler–Maruyama method, a time step of Δt = 0.1 and a system of size L × L = 625 cells) for a given set of parameters (ω, θ, μ⋆) and obtained the corresponding probability distribution \({P}({{{{\mathcal{A}}}}}_{{{{\rm{SZ}}}}})\). We then calculated the FP versus TP curve, as shown in Extended Data Fig. 8. The AUC of the FP versus TP curves for a given perturbation intensity \({{{\mathcal{I}}}}\) is referred to in the following as \({{{\rm{AUC}}}}{(\omega ,\,\theta ,\,{\mu }^{\star },\,{{{\mathcal{I}}}})}\). To simplify the comparison between parameters, we computed the average response to all the five external stimuli:
We plot the resulting values for the parameter space (ω, θ, μ⋆) in Fig. 3b. For a plot in the reduced space (ω⋆, θ, μ⋆), see Extended Data Fig. 8.
Propagation of information analysis
We studied also the propagation of the information of external stimuli in the system. For this, we used machine-learning algorithms. We generated numerical data for different values of (ω, θ, μ⋆) at a sampling rate of Δt = 0.1s, for an observation time of T = 40s and a system size of L = 25. We generated 10,000 realizations and for each one, a random perturbation time tp was selected in the interval tp ∈ [20s, 40s]. We used perturbation intensities in the interval \({{{\mathcal{I}}}}\in {[1,\,9]}\) and compared them with simulations where no perturbation was implemented. More specifically, a single realization is a sequence of 2D arrays \({{{\bf{X}}}}\in {{\mathbb{R}}}^{(T/{{\Delta }}t)\times L\times L}\), which records the dynamics of the system. For each realization, we computed an auxiliary perturbation temporary series, which is a single vector \({{{\bf{Y}}}}\in {{\mathbb{R}}}^{(T/{{\Delta }}t)}\), whose entries are 0 before the perturbation and 1 for all perturbations where the number of perturbed cells exceeds a threshold Nthresh. Hence, it is a binary classification task. For the perturbation analysis, we discarded the first 20 time units because they correspond to an initial stationary settling period. This results in a time-series of size (200, 25, 25) for a single sequence. From each of the realizations we sub-sample (without replacement) three 3 × 3 sub-patches next to the PZ. These sub-patches aim to emulate the local-agent perspective. To reduce the memory requirements of our simulations, we furthermore downsample the time dimension and only keep every second time step. The final dataset used to train the networks consists of 30,000 sub-patch sequences of shape (100, 3, 3).
LSTM training
From the numerical simulations we obtained a high-dimensional time-series of regressors and their corresponding perturbation labels across time. These arrays were reshaped into a vector format to be processed by the RNN. We first embedded Xt by using a linear layer with 128 hidden units. The embedded input patch, \({\tilde{X}}_{t}\in {{\mathbb{R}}}^{128}\), was then processed by the RNN. We used a common LSTM35 layer, which maintained a hidden state ht and a cell state ct. Given an input, these states are updated using a combination of input, forget, update and output gates:
Afterwards, the resulting hidden state ht is processed by another linear layer to provide the readout logits used to classify the presence of a perturbation. The networks are trained to optimize a simple binary cross-entropy loss using backpropagation through time (BPTT) and the Adam optimizer:
where pθ(⋅) denotes the class probabilities predicted by the LSTM. We rewrite Y as a binary one-hot encoded matrix, were c denotes a specific perturbation class. At each time step the network receives an 18D vector input. The networks were trained on 12 CPU cores each and we used the PyTorch52 automatic differentiation library. All results shown were obtained by repeating the same procedures for five independent runs with different random seeds. The technical details and parameters used for the training of the networks are listed in Extended Data Table 1.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this Article.
Data availability
All empirical data necessary to reproduce the corresponding figures and results presented in this Paper are available through the public repository Zenodo (https://doi.org/10.5281/zenodo.7323527).
Code availability
Detailed code used to train the LSTM networks is available on Github: https://github.com/RobertTLange/automata-perturbation-lstm.
References
Krause, J. & Ruxton, G. D. Living in Groups (Oxford Univ. Press, 2002).
Mora, T. & Bialek, W. Are biological systems poised at criticality? J. Stat. Phys. 144, 268–302 (2011).
Muñoz, M. A. Colloquium: criticality and dynamical scaling in living systems. Rev. Mod. Phys. 90, 031001 (2018).
Bialek, W. et al. Social interactions dominate speed control in poising natural flocks near criticality. Proc. Natl Acad. Sci. USA 111, 7212–7217 (2014).
Hesse, J. & Gross, T. Self-organized criticality as a fundamental property of neural systems. Front. Syst. Neurosci. 8, 166 (2014).
Klamser, P. P. & Romanczuk, P. Collective predator evasion: putting the criticality hypothesis to the test. PLoS Comput. Biol. 17, e1008832 (2021).
Haldeman, C. & Beggs, J. M. Critical branching captures activity in living neural networks and maximizes the number of metastable states. Phys. Rev. Lett. 94, 058101 (2005).
Levina, A., Herrmann, J. M. & Geisel, T. Dynamical synapses causing self-organized criticality in neural networks. Nat. Phys. 3, 857–860 (2007).
Beggs, J. M. The criticality hypothesis: how local cortical networks might optimize information processing. Phil. Trans. R. Soc. A 366, 329–343 (2008).
Meisel, C. & Gross, T. Adaptive self-organization in a realistic neural network model. Phys. Rev. E 80, 061917 (2009).
Friedman, N. et al. Universal critical dynamics in high resolution neuronal avalanche data. Phys. Rev. Lett. 108, 208102 (2012).
Beggs, J. & Timme, N. Being critical of criticality in the brain. Front. Physiol. 3, 163 (2012).
Priesemann, V. et al. Spike avalanches in vivo suggest a driven, slightly subcritical brain state. Front. Syst. Neurosci. 8, 108 (2014).
Bäuerle, T., Löffler, R. C. & Bechinger, C. Formation of stable and responsive collective states in suspensions of active colloids. Nat. Commun. 11, 2547 (2020).
Attanasi, A. et al. Finite-size scaling as a way to probe near-criticality in natural swarms. Phys. Rev. Lett. 113, 238102 (2014).
Doran, C. et al. Fish waves as emergent collective antipredator behavior. Curr. Biol. 32, 708–714 (2021).
Tobler, M. et al. Evolution in extreme environments: replicated phenotypic differentiation in livebearing fish inhabiting sulfidic springs. Evolution 65, 2213–2228 (2011).
Pfenninger, M. et al. Parallel evolution of cox genes in H2S-tolerant fish as key adaptation to a toxic environment. Nat. Commun. 5, 3873 (2014).
Tobler, M., Kelley, J. L., Plath, M. & Riesch, R. Extreme environments and the origins of biodiversity: adaptation and speciation in sulphide spring fishes. Mol. Ecol. 27, 843–859 (2018).
Greenway, R. et al. Convergent evolution of conserved mitochondrial pathways underlies repeated adaptation to extreme environments. Proc. Natl Acad. Sci. USA 117, 16424–16430 (2020).
Tobler, M., Riesch, R., Tobler, C. & Plath, M. Compensatory behaviour in response to sulphide-induced hypoxia affects time budgets, feeding efficiency and predation risk. Evol. Ecol. Res. 11, 935–948 (2009).
Lukas, J. et al. Diurnal changes in hypoxia shape predator-prey interaction in a bird-fish system. Front. Ecol. Evol. 9, 619193 (2021).
Riesch, R. et al. Extreme habitats are not refuges: poeciliids suffer from increased aerial predation risk in sulphidic southern Mexican habitats. Biol. J. Linnean Soc. 101, 417–426 (2010).
Lukas, J. et al. Acoustic and visual stimuli combined promote stronger responses to aerial predation in fish. Behav. Ecol. 32, 1094–1102 (2021).
Farkas, I., Helbing, D. & Vicsek, T. Mexican waves in an excitable medium. Nature 419, 131–132 (2002).
Deco, G., Jirsa, V. K. & McIntosh, A. R. Emerging concepts for the dynamical organization of resting-state activity in the brain. Nat. Rev. Neurosci. 12, 43–56 (2011).
Pizoli, C. E. et al. Resting-state activity in development and maintenance of normal brain function. Proc. Natl Acad. Sci. USA 108, 11638–11643 (2011).
Poel, W. et al. Subcritical escape waves in schooling fish. Sci. Adv. 8, eabm6385 (2022).
Wang, J., Kádár, S., Jung, P. & Showalter, K. Noise driven avalanche behavior in subexcitable media. Phys. Rev. Lett. 82, 855–858 (1999).
Clauset, A., Shalizi, C. R. & Newman, M. E. J. Power-law distributions in empirical data. SIAM Rev. 51, 661–703 (2009).
Rosenthal, S. B., Twomey, C. R., Hartnett, A. T., Wu, H. S. & Couzin, I. D. Revealing the hidden networks of interaction in mobile animal groups allows prediction of complex behavioral contagion. Proc. Natl Acad. Sci. USA 112, 4690–4695 (2015).
Kinouchi, O. & Copelli, M. Optimal dynamical range of excitable networks at criticality. Nat. Phys. 2, 348–351 (2006).
Cavagna, A. et al. Scale-free correlations in starling flocks. Proc. Natl Acad. Sci. USA 107, 11865–11870 (2010).
Calovi, D. S. et al. Collective response to perturbations in a data-driven fish school model. J. R. Soc. Interface 12, 20141362 (2015).
Hochreiter, S. & Schmidhuber, J. Long short-term memory. Neural Comput. 9, 1735–1780 (1997).
Drossel, B. & Schwabl, F. Self-organized critical forest-fire model. Phys. Rev. Lett. 69, 1629–1632 (1992).
Grassberger, P. Critical behaviour of the Drossel-Schwabl forest fire model. New J. Phys. 4, 17 (2002).
Biswas, S., Chandra, A. K., Chatterjee, A. & Chakrabarti, B. K. Phase transitions and non-equilibrium relaxation in kinetic models of opinion formation. J. Phys. Conf. Ser. 297, 012004 (2011).
Hidalgo, J. et al. Information-based fitness and the emergence of criticality in living systems. Proc. Natl Acad. Sci. USA 111, 10095–10100 (2014).
Bornholdt, S. & Rohlf, T. Topological evolution of dynamical networks: global criticality from local dynamics. Phys. Rev. Lett. 84, 6114–6117 (2000).
Mora, T. et al. Local equilibrium in bird flocks. Nat. Phys. 12, 1153–1157 (2016).
Daniels, B. C., Krakauer, D. C. & Flack, J. C. Control of finite critical behaviour in a small-scale social system. Nat. Commun. 8, 14301 (2017).
Sosna, M. M. G. et al. Individual and collective encoding of risk in animal groups. Proc. Natl Acad. Sci. USA 116, 20556–20561 (2019).
Jolles, J. W., Boogert, N. J., Sridhar, V. H., Couzin, I. D. & Manica, A. Consistent individual differences drive collective behavior and group functioning of schooling fish. Curr. Biol. 27, 2862–2868 (2017).
Rahmani, P., Peruani, F. & Romanczuk, P. Flocking in complex environments-attention trade-offs in collective information processing. PLoS Comput. Biol. 16, e1007697 (2020).
Cramer, B. et al. Control of criticality and computation in spiking neuromorphic networks with plasticity. Nat. Commun. 11, 2853 (2020).
OpenCV: Open Source Computer Vision Library; https://opencv.org/
Background Subtraction Methods in Python using OpenCV; https://docs.opencv.org/3.4/d1/dc5/tutorial_background_subtraction.html
Alstott, J., Bullmore, E. & Plenz, D. powerlaw: a Python package for analysis of heavy-tailed distributions. PLoS ONE 9, e85777 (2014).
Lindner, B., García-Ojalvo, J., Neiman, A. & Schimansky-Geier, L. Effects of noise in excitable systems. Phys. Rep. 392, 321–424 (2004).
Press, W. H., Teukolsky, S. A., Vetterling, W. T. & Flannery, B. P. Numerical Recipes in C: The Art of Scientific Computing (Cambridge Univ. Press, 2007).
Paszke, A. et al. PyTorch: an imperative style, high-performance deep learning library. In Advances in Neural Information Processing Systems (eds Wallach H. et al.) 8026–8037 (Curran Associates Inc., 2019).
Acknowledgements
We are grateful to the director and staff at the Centro de Investigación e Innovación para la Enseñanza y el Aprendizaje (CIIEA) field station in Teapa (Mexico) for hosting our multiple research stays. In addition, we thank B. Lindner and I. Sokolov for helpful discussions and advice, and H. Klenz for support for the acquisition of data. The acquisition of videos adhered to the ‘Guidelines for the treatment of animals in behavioral research and teaching’ (Animal Behaviour 2021) and were approved by the Mexican government (DGOPA.09004.041111.3088, PRMN/DGOPA-009/2015 and PRMN/DGOPA-012/2017 issued by SAGARPA-CONAPESCA-DGOPA). This work was supported by the Deutsche Forschungsgemeinschaft (German Research Foundation): BI 1828/3-1 (J.L. and D.B.), RO 4766/2-1 (P.R.) and under Germany’s Excellence Strategy - EXC 2002/1 ‘Science of Intelligence’ project- no. 390523135 (L.G.-N., R.T.L., D.B., J.K., H.S. and P.R.). J.L. was partially supported by the Berlin Funding for Graduates (Elsa-Neumann-Scholarship des Landes Berlin).
Author information
Authors and Affiliations
Contributions
L.G.-N., R.T.L., H.S. and P.R. designed the study. P.P.K., J.L., L.A.R., D.B., J.K. and P.R. acquired and processed the empirical data. L.G.-N. analysed the empirical data. L.G.-N. and R.T.L. performed the numerical simulations. L.G.-N., R.T.L., H.S. and P.R. analysed the results. L.G.-N., R.T.L., D.B., H.S. and P.R. wrote the paper. All authors commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Physics thanks Clément Sire and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Goodness-of-fit of the characteristic times distributions.
a, Distribution P(D) generated with synthetic data from a gamma distribution (eq. (4)) with parameters κ = 0.59 and Ω = 21.72. D is the Kolmogorov-Smirnov distance of the empirical (Demp) or synthetically generated (D) data to the theoretical distribution. The parameters κ and Ω were fitted using the empirical values of the inter-spike times τ1. The p-value results to be p = 0.015. b, A similar procedure was applied to the spike-duration times τ2, where the obtained numerical values of the parameters are κ = 0.69 and Ω = 0.99. In this case, the p-value results to be p = 0.027.
Extended Data Fig. 2 Power-law analysis of the empirical cluster distributions.
a,b, Plots of the cluster areas distribution P(a) and the cluster volumes distribution P(v). In each plot, we present the distribution of KS distances P(D) obtained from the synthetic data that served us to calculate the corresponding p-values. The p-value for the cluster areas results to be p=0.044, and for the cluster volumes we obtain p=0.0056. c, Plot of the information of each cluster: area, speed, time-duration and volume.
Extended Data Fig. 3 Numerical implementation of the model and parameter selection.
a, Snapshot of the numerical simulations of a system of size L = 25. The cells can adopt three different states, depicted with three colors: white (state \({{{\mathcal{S}}}}\)), red (state \({{{\mathcal{D}}}}\)) and blue (state \({{{\mathcal{U}}}}\)). b,c, Scheme of the interaction neighborhood of a given cell ij and the absorbing boundary conditions used for the numerical simulations. Each cell is affected by its 8 nearest neighbors (Moore neighborhood). d,e, Plot of the interaction term of the transition rate given in equation (15) for constant neighbor coupling θ and constant parameter μ respectively. f,g, Plot of the auxiliary functions ϵ1(ω, θ, μ) and ϵ2(ω, θ, μ) for μ = μ⋆=1. In each plot, the orange line denotes the region where the auxiliary functions are minimized. The crossing of both lines is denoted by blue arrows. h, Plot of ϵ (ω⋆, θ⋆, μ) for different values of μ. The minimal value is reached at μ⋆=1.
Extended Data Fig. 4 Results from the analysis of the empirical surface-activity signals.
a-c, Numerical values of the three characteristic quantities \({\left\langle \mathcal{A}\right\rangle }_{{{{\rm{t}}}}},{\mathcal{A}}_{{{{\rm{th}}}}}\) and \({\mathcal{R}}\) that we used to analyse the surface-activity signals. The grey-dashed vertical lines divide the data by days of acquisition on the field station. In c, we also show the mean value of the ratio \({\mathcal{R}}_{\mathrm{emp}}\) calculated over all videos.
Extended Data Fig. 5 Average number of active neighbors.
Scheme of the computation of the average number of active neighbor cells for a given set of focal cells. The times ti represent the randomly selected times at which a set of 8 focal cells are randomly selected. The focal cells are depicted in red. We computed the number of active neighboring cells for each focal cell and averaged over all focal cells and over all randomly selected times ti. The value obtained from the empirical videos is \({\mathcal{N}}\)emp = 0.27 ± 0.23.
Extended Data Fig. 6 Study of the critical transition.
a, Susceptibility computed for six different system sizes LWϵ [25, 50, 100, 200, 400, 800] and for different values of the neighbor coupling parameter θ. b, Value of the susceptibility in three different regimes: i) θ = θc, ii) θ < θc and iii) θ > θc, where θc is the value of the neighbor coupling parameter θ where the susceptibility reaches its maximum value. c, Width of the susceptibility peak \({\mathcal{W}}\) - as defined in equation (18) - for different system sizes L.
Extended Data Fig. 7 Susceptibility in empirical data.
Susceptibility computed as defined in equation (17) using the empirical videos and five different window sizes LWϵ [25, 50, 100, 200, 400] pixels. The results of the analysis for the different window sizes of each of the videos are connected by a gray dashed line. The average susceptibility over videos is plotted as a single red square for each window size LW. The best power-law fit to the average values is shown as a black line and the label shows the exponent that fits best the data. The data is shown separately for all acquisition days. In all cases, the best power-law fit has an exponent that is between 1 and 2.
Extended Data Fig. 8 Susceptibility analysis.
Plots of the true positive (TP) vs true negative (TN) curves for three different values of the coupling parameter θ, as well as the plot of the average response to stimuli ϕAUC(ω⋆, θ, μ⋆) for different values of θ.
Supplementary information
Supplementary Video 1
Close up video of the behaviour of the sulphur mollies taken underwater. The video shows a sequence where the fish swim very close to the surface of the water, followed by a dive of all the individuals. This highly synchronized collective diving behaviour can be traced from outside of the water (visual splash observed in Supplementary Video 2) and is referred to in the main text as surface wave activity. Video taken by Juliane Lukas and Pawel Romanczuk.
Supplementary Video 2
Unprocessed raw video of the surface wave activity observed in the system. The total area of the water surface is ~600 m2. Video taken by Pascal P. Klamser and Pawel Romanczuk.
Supplementary Video 3
Processed video used to characterize the surface wave activity observed in the system. The video was first rectified to reconstruct a top view of the pond, and then converted into a black-and-white video using the background subtraction process (MOG2 OpenCV). For details, see Methods.
Supplementary Video 4
Dynamics observed in the three regimes: (1) θ < θc, (2) θ = θc and (3) θ > θc.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gómez-Nava, L., Lange, R.T., Klamser, P.P. et al. Fish shoals resemble a stochastic excitable system driven by environmental perturbations. Nat. Phys. 19, 663–669 (2023). https://doi.org/10.1038/s41567-022-01916-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41567-022-01916-1