Abstract
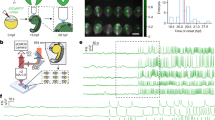
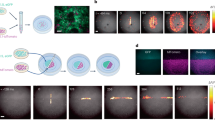
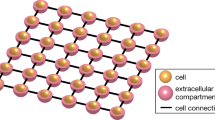
Electrical signalling in biology is typically associated with action potentials—transient spikes in membrane voltage that return to baseline. Hodgkin–Huxley and related conductance-based models of electrophysiology belong to a more general class of reaction–diffusion equations that could, in principle, support the spontaneous emergence of patterns of membrane voltage that are stable in time but structured in space. Here, we show theoretically and experimentally that homogeneous or nearly homogeneous tissues can undergo spontaneous spatial symmetry breaking through a purely electrophysiological mechanism, leading to the formation of domains with different resting potentials separated by stable bioelectrical domain walls. Transitions from one resting potential to another can occur through long-range migration of these domain walls. We map bioelectrical domain wall motion using all-optical electrophysiology in an engineered cell line and in human induced pluripotent stem cell (iPSC)-derived myoblasts. Bioelectrical domain wall migration may occur during embryonic development and during physiological signalling processes in polarized tissues. These results demonstrate that nominally homogeneous tissues can undergo spontaneous bioelectrical spatial symmetry breaking.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The plots of fluorescent voltage recordings in Figs. 2–4 are available as Source Data. All other data that support the plots within this paper and other findings of this study are available from the corresponding author upon reasonable request.
Code availability
Custom-written code used for data analysis is available from the authors upon request.
References
Hodgkin, A. L. & Huxley, A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J. Physiol. 117, 500–544 (1952).
Turing, A. M. The chemical basis of morphogenesis. Philos. Trans. R. Soc. Lond. B 237, 37–72 (1952).
Kondo, S. & Miura, T. Reaction–diffusion model as a framework for understanding biological pattern formation. Science 329, 1616–1620 (2010).
Law, R. & Levin, M. Bioelectric memory: modeling resting potential bistability in amphibian embryos and mammalian cells. Theor. Biol. Med. Model. 12, 22 (2015).
Pietak, A. & Levin, M. Bioelectric gene and reaction networks: computational modelling of genetic, biochemical and bioelectrical dynamics in pattern regulation. J. R. Soc. Interface 14, 20170425 (2017).
Cervera, J., Alcaraz, A. & Mafe, S. Bioelectrical signals and ion channels in the modeling of multicellular patterns and cancer biophysics. Sci. Rep. 6, 20403 (2016).
Brodsky, M. Turing-like patterns can arise from purely bioelectric mechanisms. Preprint at https://www.biorxiv.org/content/10.1101/336461v1.full (2018).
Levin, M. Endogenous bioelectrical networks store non‐genetic patterning information during development and regeneration. J. Physiol. 592, 2295–2305 (2014).
Hochbaum, D. R. et al. All-optical electrophysiology in mammalian neurons using engineered microbial rhodopsins. Nat. Methods 11, 825–833 (2014).
Adam, Y. et al. Voltage imaging and optogenetics reveal behaviour-dependent changes in hippocampal dynamics. Nature 569, 413 (2019).
ten Tusscher, K. H. W. J., Noble, D., Noble, P. J. & Panfilov, A. V. A model for human ventricular tissue. Am. J. Physiol. Heart Circ. Physiol. 286, H1573–H1589 (2004).
Hibino, H. et al. Inwardly rectifying potassium channels: their structure, function and physiological roles. Physiol. Rev. 90, 291–366 (2010).
Cervera, J., Alcaraz, A. & Mafe, S. Membrane potential bistability in nonexcitable cells as described by inward and outward voltage-gated ion channels. J. Phys. Chem. B 118, 12444–12450 (2014).
Gallaher, J., Bier, M. & van Heukelom, J. S. First order phase transition and hysteresis in a cell’s maintenance of the membrane potential—an essential role for the inward potassium rectifiers. BioSystems 101, 149–155 (2010).
Ozbudak, E. M., Thattai, M., Lim, H. N., Shraiman, B. I. & Van Oudenaarden, A. Multistability in the lactose utilization network of Escherichia coli. Nature 427, 737 (2004).
Novick, A. & Weiner, M. Enzyme induction as an all-or-none phenomenon. Proc. Natl Acad. Sci. USA 43, 553–566 (1957).
McNamara, H. M., Zhang, H., Werley, C. A. & Cohen, A. E. Optically controlled oscillators in an engineered bioelectric tissue. Phys. Rev. X 6, 031001 (2016).
McNamara, H. M. et al. Geometry-dependent arrhythmias in electrically excitable tissues. Cell Syst. 7, 359–370 (2018).
Huang, Y. L., Walker, A. S. & Miller, E. W. A photostable silicon rhodamine platform for optical voltage sensing. J. Am. Chem. Soc. 137, 10767–10776 (2015).
Cervera, J., Manzanares, J. A. & Mafe, S. Electrical coupling in ensembles of nonexcitable cells: modeling the spatial map of single cell potentials. J. Phys. Chem. B 119, 2968–2978 (2015).
Benguria, R. & Depassier, M. Speed of fronts of the reaction-diffusion equation. Phys. Rev. Lett. 77, 1171 (1996).
Cohen, A. E. Nanoscale Mechanics. PhD thesis, Univ. Cambridge (2004).
Moreno, A., Rook, M., Fishman, G. & Spray, D. C. Gap junction channels: distinct voltage-sensitive and -insensitive conductance states. Biophys. J. 67, 113–119 (1994).
Butterweck, A., Gergs, U., Elfgang, C., Willecke, K. & Traub, O. Immunochemical characterization of the gap junction protein connexin45 in mouse kidney and transfected human HeLa cells. J. Membr. Biol. 141, 247–256 (1994).
Langlois, S., Cowan, K. N., Shao, Q., Cowan, B. J. & Laird, D. W. Caveolin-1 and-2 interact with connexin43 and regulate gap junctional intercellular communication in keratinocytes. Mol. Biol. Cell 19, 912–928 (2008).
Barkhausen, H. Zwei mit Hilfe der neuen Verstärker entdeckte Erscheinungen. Phys. Z 20, 401 (1919).
Cross, M. H., Cross, P. & Brinster, R. Changes in membrane potential during mouse egg development. Dev. Biol. 33, 412–416 (1973).
Liu, J. et al. Role of an inward rectifier K current and of hyperpolarization in human myoblast fusion. J. Physiol. 510, 467–476 (1998).
Konig, S. et al. Membrane hyperpolarization triggers myogenin and myocyte enhancer factor-2 expression during human myoblast differentiation. J. Biol. Chem. 279, 28187–28196 (2004).
Kalderon, N., Epstein, M. L. & Gilula, N. B. Cell-to-cell communication and myogenesis. J. Cell Biol. 75, 788–806 (1977).
Metcalf, M. M., Shi, R. & Borgens, R. B. Endogenous ionic currents and voltages in amphibian embryos. J. Exp. Zool. 268, 307–322 (1994).
Borgens, R. B., Rouleau, M. F. & DeLanney, L. E. A steady efflux of ionic current predicts hind limb development in the axolotl. J. Exp. Zool. 228, 491–503 (1983).
Whited, J. L. & Levin, M. Bioelectrical controls of morphogenesis: from ancient mechanisms of cell coordination to biomedical opportunities. Curr. Opin. Genet. Dev. 57, 61–69 (2019).
Rinne, P. L. & van der Schoot, C. Symplasmic fields in the tunica of the shoot apical meristem coordinate morphogenetic events. Development 125, 1477–1485 (1998).
Achenbach, F. & Weisenseel, M. Ionic currents traverse the slime mould Physarum. Cell Biol. Int. Rep. 5, 375–379 (1981).
Zhao, M. Electrical fields in wound healing—an overriding signal that directs cell migration. Semin. Cell. Dev. Biol. 20, 674–682 (2009).
Borgens, R. B. Endogenous ionic currents traverse intact and damaged bone. Science 225, 478–482 (1984).
Zhou, Y. et al. Membrane potential modulates plasma membrane phospholipid dynamics and K-Ras signaling. Science 349, 873–876 (2015).
Gurdon, J., Tiller, E., Roberts, J. & Kato, K. A community effect in muscle development. Curr. Biol. 3, 1–11 (1993).
Proulx, A., Merrifield, P. A. & Naus, C. C. Blocking gap junctional intercellular communication in myoblasts inhibits myogenin and MRF4 expression. Dev. Genet. 20, 133–144 (1997).
Crunelli, V., Tóth, T. I., Cope, D. W., Blethyn, K. & Hughes, S. W. The ‘window’ T‐type calcium current in brain dynamics of different behavioural states. J. Physiol. 562, 121–129 (2005).
Hughes, S. W., Cope, D. W., Toth, T. I., Williams, S. R. & Crunelli, V. All thalamocortical neurones possess a T‐type Ca2+ ‘window’ current that enables the expression of bistability‐mediated activities. J. Physiol. 517, 805–815 (1999).
Crill, W. E. Persistent sodium current in mammalian central neurons. Annu. Rev. Physiol. 58, 349–362 (1996).
Li, Y. & Bennett, D. J. Persistent sodium and calcium currents cause plateau potentials in motoneurons of chronic spinal rats. J. Neurophysiol. 90, 857–869 (2003).
Schwindt, P. C. & Crill, W. E. Amplification of synaptic current by persistent sodium conductance in apical dendrite of neocortical neurons. J. Neurophysiol. 74, 2220–2224 (1995).
Constantin, B. & Cronier, L. Involvement of gap junctional communication in myogenesis. Int. Rev. Cytol. 196, 1–65 (2000).
Levin, M. Isolation and community: a review of the role of gap-junctional communication in embryonic patterning. J. Membr. Biol. 185, 177–192 (2002).
Mobasheri, A. et al. Potassium channels in articular chondrocytes. Channels 6, 416–425 (2012).
Barrett-Jolley, R., Lewis, R., Fallman, R. & Mobasheri, A. The emerging chondrocyte channelome. Front. Physiol. 1, 135 (2010).
Tecott, L., Shtrom, S. & Julius, D. Expression of a serotonin-gated ion channel in embryonic neural and nonneural tissues. Mol. Cell. Neurosci. 6, 43–55 (1995).
Chifflet, S., Hernández, J. A. & Grasso, S. A possible role for membrane depolarization in epithelial wound healing. Am. J. Physiol. Cell Physiol. 288, C1420–C1430 (2005).
Ayata, C. & Lauritzen, M. Spreading depression, spreading depolarizations and the cerebral vasculature. Physiol. Rev. 95, 953–993 (2015).
FitzHugh, R. Impulses and physiological states in theoretical models of nerve membrane. Biophys. J. 1, 445–466 (1961).
Nagumo, J., Arimoto, S. & Yoshizawa, S. An active pulse transmission line simulating nerve axon. Proc. IRE 50, 2061–2070 (1962).
Onsager, L. Crystal statistics. I. A two-dimensional model with an order-disorder transition. Phys. Rev. 65, 117 (1944).
Zeldovich, Y. & Frank-Kamenetskii, D. Theory of flame propagation. Zh. Fix. Khim 12, 100 (1938).
Allee, W. C., Park, O., Emerson, A. E., Park, T. & Schmidt, K. P. Principles of Animal Ecology (W. B. Saunders, 1949).
Coombes, S. Waves, bumps and patterns in neural field theories. Biol. Cybern. 93, 91–108 (2005).
Shiferaw, Y. & Karma, A. Turing instability mediated by voltage and calcium diffusion in paced cardiac cells. Proc. Natl Acad. Sci. USA 103, 5670–5675 (2006).
Echebarria, B. & Karma, A. Instability and spatiotemporal dynamics of alternans in paced cardiac tissue. Phys. Rev. Lett. 88, 208101 (2002).
Chal, J. et al. Generation of human muscle fibers and satellite-like cells from human pluripotent stem cells in vitro. Nat. Protoc. 11, 1833 (2016).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Trapnell, C. et al. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 28, 511–515 (2010).
Cornwell, M. et al. VIPER: visualization pipeline for RNA-seq, a snakemake workflow for efficient and complete RNA-seq analysis. BMC Bioinformatics 19, 135 (2018).
Werley, C. A., Chien, M. & Cohen, A. E. Ultrawidefield microscope for high-speed fluorescence imaging and targeted optogenetic stimulation. Biomed. Opt. Express 8, 5794–5813 (2017).
Acknowledgements
We thank U. Böhm, A. Klaeger, J. Mathews and M. Levin for helpful discussions. We thank E. Miller for help providing the BeRST1 dye. This work was supported by the Allen Discovery Center at Tufts University, the Vannevar Bush Fellowship Foundation and the Howard Hughes Medical Institute. H.M.M. was supported by the Department of Defense (DoD) through the National Defense Science & Engineering Graduate Fellowship (NDSEG) Program. G.O. was supported by the Howard Hughes Medical Institute Gilliam Fellowship.
Author information
Authors and Affiliations
Contributions
H.M.M. and A.E.C. conceived and designed the study. H.M.M. conducted experiments and analysed results, with assistance from R.S., H.X. and S.B. H.M.M. designed and simulated numerical models of electrically bistable cells. Z.A.T. and O.P. provided hiPSC-derived myoblasts for myocyte differentiation and characterized these cells via immunocytochemistry and RNA-seq. G.O. provided BeRST1 dye reagent. H.M.M. and A.E.C. wrote the manuscript, with input from Z.A.T. and O.P. A.E.C. and O.P. oversaw the research.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Physics thanks Salvador Mafe, Min Zhao and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–9, Tables 1 and 2, and video captions 1–6.
Supplementary Video 1
Simulation of nucleation and growth of bioelectrical domains in homogeneous tissue.
Supplementary Video 2
Nucleation and growth of bioelectrical domains in a confluent culture of bi-HEK cells, example 1.
Supplementary Video 3
Nucleation and growth of bioelectrical domains in a confluent culture of bi-HEK cells, example 2.
Supplementary Video 4
Switching of discrete bistable bioelectrical domains in disordered culture of bi-HEK cells.
Supplementary Video 5
Simulation of switching of discrete bistable bioelectrical domains in disordered tissue.
Supplementary Video 6
Depolarization via domain wall propagation in a confluent culture of human iPSC-derived myocytes.
Source data
Source Data Fig. 2
Fluorescence vs. time for Fig. 2e
Source Data Fig. 3
Fluorescence vs. time for Fig. 3f
Source Data Fig. 4
Fluorescence vs. time for Fig. 4c,g
Rights and permissions
About this article
Cite this article
McNamara, H.M., Salegame, R., Tanoury, Z.A. et al. Bioelectrical domain walls in homogeneous tissues. Nat. Phys. 16, 357–364 (2020). https://doi.org/10.1038/s41567-019-0765-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41567-019-0765-4
This article is cited by
-
Genetically engineered HEK cells as a valuable tool for studying electroporation in excitable cells
Scientific Reports (2024)
-
Electron transfer in protein modifications: from detection to imaging
Science China Chemistry (2023)
-
Observation of topological action potentials in engineered tissues
Nature Physics (2022)
-
A bioelectric model of carcinogenesis, including propagation of cell membrane depolarization and reversal therapies
Scientific Reports (2021)