Abstract
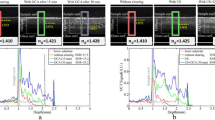
Although laser scanning microscopy is a pivotal imaging tool in biomedical research, optical scattering from tissue limits the depth of the imaging. To overcome this limitation, we propose a scheme called ultrasound-induced optical clearing microscopy, which makes use of temporary, localized optical clearing based on ultrasound-induced gas bubbles. In this method, bubbles are generated by high-intensity pulsed ultrasound at a desired depth and subsequently maintained by low-intensity continuous ultrasound during imaging. As a result, optical scattering and unwanted changes in the propagation direction of the incident photons are minimized in the bubble cloud, and thus the laser can be tightly focused at a deeper imaging plane. Through phantom and ex vivo experiments, we demonstrate that ultrasound-induced optical clearing microscopy is capable of increasing the imaging depth by a factor of six or more, while the resolution is similar to that of conventional laser scanning microscopy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding authors upon reasonable request.
Code availability
The codes for the simulation are available from the corresponding authors upon reasonable request.
References
Pawley, J. B. (ed.) Handbook of Biological Confocal Microscopy 3rd edn (Springer, 2006); https://doi.org/10.1007/978-0-387-45524-2
Ntziachristos, V. Going deeper than microscopy: the optical imaging frontier in biology. Nat. Methods 7, 603–614 (2010).
Yoon, S. et al. Deep optical imaging within complex scattering media. Nat. Rev. Phys. 2, 141–158 (2020).
Benninger, R. K. P. & Piston, D. W. Two-photon excitation microscopy for the study of living cells and tissues. Curr. Protoc. Cell Biol. 59, 4.11.1–4.11.24 (2013).
Yu, H. et al. Recent advances in wavefront shaping techniques for biomedical applications. Curr. Appl. Phys. 15, 632–641 (2015).
Horstmeyer, R., Ruan, H. & Yang, C. Guidestar-assisted wavefront-shaping methods for focusing light into biological tissue. Nat. Photonics 9, 563–571 (2015).
Jang, M. et al. Deep tissue space-gated microscopy via acousto-optic interaction. Nat. Commun. 11, 710 (2020).
Omar, M., Aguirre, J. & Ntziachristos, V. Optoacoustic mesoscopy for biomedicine. Nat. Biomed. Eng. 3, 354–370 (2019).
Zhang, Z. et al. Imaging volumetric dynamics at high speed in mouse and zebrafish brain with confocal light field microscopy. Nat. Biotechnol. 39, 74–83 (2021).
Xu, X., Liu, H. & Wang, L. V. Time-reversed ultrasonically encoded optical focusing into scattering media. Nat. Photonics 5, 154–157 (2011).
Wang, Y. M., Judkewitz, B., DiMarzio, C. A. & Yang, C. Deep-tissue focal fluorescence imaging with digitally time-reversed ultrasound-encoded light. Nat. Commun. 3, 928 (2012).
Si, K., Fiolka, R. & Cui, M. Fluorescence imaging beyond the ballistic regime by ultrasound-pulse-guided digital phase conjugation. Nat. Photonics 6, 657–661 (2012).
Ruan, H., Liu, Y., Xu, J., Huang, Y. & Yang, C. Fluorescence imaging through dynamic scattering media with speckle-encoded ultrasound-modulated light correlation. Nat. Photonics 14, 511–516 (2020).
Park, J.-H., Yu, Z., Lee, K., Lai, P. & Park, Y. Perspective: wavefront shaping techniques for controlling multiple light scattering in biological tissues: toward in vivo applications. APL Photonics 3, 100901 (2018).
Zhu, X. et al. Ultrafast optical clearing method for three-dimensional imaging with cellular resolution. Proc. Natl Acad. Sci. USA 116, 11480–11489 (2019).
Costantini, I., Cicchi, R., Silvestri, L., Vanzi, F. & Pavone, F. S. In-vivo and ex-vivo optical clearing methods for biological tissues: review. Biomed. Opt. Express 10, 5251–5267 (2019).
Yu, T., Zhu, J., Li, D. & Zhu, D. Physical and chemical mechanisms of tissue optical clearing. iScience 24, 102178 (2021).
Jin, Y. L., Chen, J. Y., Xu, L. & Wang, P. N. Refractive index measurement for biomaterial samples by total internal reflection. Phys. Med. Biol. 51, N371–N379 (2006).
Hsieh, Z.-H., Fan, C.-H., Ho, Y.-J., Li, M.-L. & Yeh, C.-K. Improvement of light penetration in biological tissue using an ultrasound-induced heating tunnel. Sci Rep. 10, 17406 (2020).
Laufer, J., Simpson, R., Kohl, M., Essenpreis, M. & Cope, M. Effect of temperature on the optical properties of ex vivo human dermis and subdermis. Phys. Med. Biol. 43, 2479–2489 (1998).
Su, Y. et al. Measurements of the thermal coefficient of optical attenuation at different depth regions of in vivo human skins using optical coherence tomography: a pilot study. Biomed. Opt. Express 6, 500–513 (2015).
Shehata, I. A. Treatment with high intensity focused ultrasound: secrets revealed. Eur. J. Radiol. 81, 534–541 (2012).
Ilovitsh, T. et al. Enhanced microbubble contrast agent oscillation following 250 kHz insonation. Sci Rep. 8, 16347 (2018).
Maxwell, A. D., Cain, C. A., Hall, T. L., Fowlkes, J. B. & Xu, Z. Probability of cavitation for single ultrasound pulses applied to tissues and tissue-mimicking materials. Ultrasound Med. Biol. 39, 449–465 (2013).
Vlaisavljevich, E. et al. Effects of tissue stiffness, ultrasound frequency, and pressure on histotripsy-induced cavitation bubble behavior. Phys. Med. Biol. 60, 2271–2292 (2015).
Hoogenboom, M. et al. Mechanical high-intensity focused ultrasound destruction of soft tissue: working mechanisms and physiologic effects. Ultrasound Med. Biol. 41, 1500–1517 (2015).
Lin, K.-W. et al. Histotripsy beyond the intrinsic cavitation threshold using very short ultrasound pulses: microtripsy. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 61, 251–0885 (2014).
Şen, T., Tüfekçioğlu, O. & Koza, Y. Mechanical index. Anatol. J. Cardiol. 15, 334–336 (2015).
Marti, D., Aasbjerg, R. N., Andersen, P. E. & Hansen, A. K. MCmatlab: an open-source, user-friendly, MATLAB-integrated three-dimensional Monte Carlo light transport solver with heat diffusion and tissue damage. J. Biomed. Opt. 23, 121622 (2018).
Kim, O. A., Ohmae, S. & Medina, J. F. A cerebello-olivary signal for negative prediction error is sufficient to cause extinction of associative motor learning. Nat. Neurosci. 23, 1550–1554 (2020).
McLaughlan, J., Rivens, I., Leighton, T. & ter Haar, G. A study of bubble activity generated in ex vivo tissue by high intensity focused ultrasound. Ultrasound Med. Biol. 36, 1327–1344 (2010).
Nagarajan, V. K. & Yu, B. Monitoring of tissue optical properties during thermal coagulation of ex vivo tissues. Lasers Surg. Med. 48, 686–694 (2016).
Kim, H. & Chang, J. H. Increased light penetration due to ultrasound-induced air bubbles in optical scattering media. Sci Rep. 7, 16105 (2017).
Vaezy, S. et al. Real-time visualization of high-intensity focused ultrasound treatment using ultrasound imaging. Ultrasound Med. Biol. 27, 33–42 (2001).
Kim, J., Kim, H. & Chang, J. H. Endoscopic probe for ultrasound-assisted photodynamic therapy of deep-lying tissue. IEEE Access 8, 179745–179753 (2020).
Prentice, P., Cuschieri, A., Dholakia, K., Prausnitz, M. & Campbell, P. Membrane disruption by optically controlled microbubble cavitation. Nat. Phys. 1, 107–110 (2005).
Fan, Q., Hu, W. & Ohta, A. T. Laser-induced microbubble poration of localized single cells. Lab Chip 14, 1572–1578 (2014).
Mao, M., Montgomery, J. M., Kubke, M. F. & Thorne, P. R. The structural development of the mouse dorsal cochlear nucleus. J. Assoc. Res. Otolaryngol. 16, 473–486 (2015).
Tario, J. D. Jr et al. Optimized staining and proliferation modeling methods for cell division monitoring using cell tracking dyes. J. Vis. Exp. e4287 (2012); https://doi.org/10.3791/4287
Acknowledgements
This work was supported by the Samsung Research Funding Center of Samsung Electronics under project number SRFC-IT1702-03 (J.H.C. and J.Y.W.).
Author information
Authors and Affiliations
Contributions
J.H.C. conceived the idea, and J.H.C. and J.Y.H. developed the idea and designed the project. H.K., J.Y.H. and J.H.C. developed the experimental protocol. S.Y., J.K. and M.L. designed and built the US-OCM system. S.P. performed the ultrasound beam simulations and analysed the standing-wave properties. H.K. collected the experimental data, performed the Monte Carlo simulations and wrote the initial manuscript draft. J.H.C. and J.Y.H. revised the draft. All authors were involved in the analysis of the results and revision of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Photonics thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary description of the developed system, simulation and experimental results, discussion and Figs. 1–15.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kim, H., Youn, S., Kim, J. et al. Deep laser microscopy using optical clearing by ultrasound-induced gas bubbles. Nat. Photon. 16, 762–768 (2022). https://doi.org/10.1038/s41566-022-01068-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41566-022-01068-x
This article is cited by
-
Multimodal optical clearing to minimize light attenuation in biological tissues
Scientific Reports (2023)
-
Bubbles clear the way for imaging
Nature Photonics (2022)