Abstract
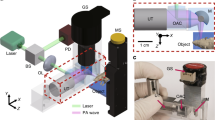
Current embodiments of photoacoustic imaging require either serial detection with a single-element ultrasonic transducer or parallel detection with an ultrasonic array, necessitating a trade-off between cost and throughput. Here, we present photoacoustic topography through an ergodic relay (PATER) for low-cost high-throughput snapshot wide-field imaging. Encoding spatial information with randomized temporal signatures through ergodicity, PATER requires only a single-element ultrasonic transducer to capture a wide-field image with a single laser shot. We applied PATER to demonstrate both functional imaging of haemodynamic responses and high-speed imaging of blood pulse wave propagation in mice in vivo. Leveraging the high frame rate of 2 kHz, PATER tracked and localized moving melanoma tumour cells in the mouse brain in vivo, which enabled flow velocity quantification and super-resolution imaging. Among the potential biomedical applications of PATER, wearable devices to monitor human vital signs in particular is envisaged.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data that support the plots within this paper and other findings of this study are available from the corresponding author upon reasonable request and with permission from corporate collaborations.
Code availability
The reconstruction algorithm and data processing methods are described in detail in the Methods. We have opted not to make the computer codes publicly available owing to corporate collaborations and pending patent applications.
References
Juskaitis, R., Wilson, T., Neil, M. M. A. & Kozubek, M. Efficient real-time confocal microscopy with white light sources. Nature 383, 804–806 (1996).
Denk, W., Strickler, J. H. & Webb, W. W. Two-photon laser scanning fluorescence microscopy. Science 248, 73–76 (1990).
Horton, N. G. et al. In vivo three-photon microscopy of subcortical structures within an intact mouse brain. Nat. Photon. 7, 205–209 (2013).
Vakoc, B. J., Fukumura, D., Jain, R. K. & Bouma, B. E. Cancer imaging by optical coherence tomography: preclinical progress and clinical potential. Nat. Rev. Cancer 12, 363–368 (2012).
Etoh, T. G. et al. An image sensor which captures 100 consecutive frames at 1000000 frames/s. IEEE Trans. Electron. Dev. 50, 144–151 (2003).
Gao, L., Liang, J., Li, C. & Wang, L. V. Single-shot compressed ultrafast photography at one hundred billion frames per second. Nature 516, 74–77 (2014).
Wu, H. et al. Eulerian video magnification for revealing subtle changes in the world. ACM Trans. Graphics 31, 1–8 (2012).
Bouchard, M. B., Chen, B. R., Burgess, S. A. & Hillman, E. M. C. Ultra-fast multispectral optical imaging of cortical oxygenation, blood flow, and intracellular calcium dynamics. Opt. Express 17, 15670–15678 (2009).
Wang, L. V. & Hu, S. Photoacoustic tomography: in vivo imaging from organelles to organs. Science 335, 1458–1462 (2012).
Jathoul, A. P. et al. Deep in vivo photoacoustic imaging of mammalian tissues using a tyrosinase-based genetic reporter. Nat. Photon. 9, 239–246 (2015).
Taruttis, A. & Ntziachristos, V. Advances in real-time multispectral optoacoustic imaging and its applications. Nat. Photon. 9, 219–227 (2015).
Wang, L. V. & Yao, J. A practical guide to photoacoustic tomography in the life sciences. Nat. Methods 13, 627–638 (2016).
Li, L. et al. Single-impulse panoramic photoacoustic computed tomography of small-animal whole-body dynamics at high spatiotemporal resolution. Nat. Biomed. Eng. 1, 0071 (2017).
Deán-Ben, X. L. & Razansky, D. Adding fifth dimension to optoacoustic imaging: volumetric time-resolved spectrally enriched tomography. Light Sci. Appl. 3, e137 (2014).
Yao, J. et al. Multiscale photoacoustic tomography using reversibly switchable bacterial phytochrome as a near-infrared photochromic probe. Nat. Methods 13, 67–73 (2015).
Gamelin, J. et al. A real-time photoacoustic tomography system for small animals. Opt. Express 17, 10489–10498 (2009).
Wong, T. T. W. et al. Label-free automated three-dimensional imaging of whole organs by microtomy-assisted photoacoustic microscopy. Nat. Commun. 8, 1386 (2017).
Li, L. et al. Label-free photoacoustic tomography of whole mouse brain structures ex vivo. Neurophotonics 3, 035001 (2016).
Wang, L. V. Multiscale photoacoustic microscopy and computed tomography. Nat. Photon. 3, 503–509 (2009).
Draeger, C. & Fink, M. One-channel time reversal of elastic waves in a chaotic 2D-silicon cavity. Phys. Rev. Lett. 79, 407–410 (1997).
Ing, R. K., Quieffin, N., Catheline, S. & Fink, M. In solid localization of finger impacts using acoustic time-reversal process. Appl. Phys. Lett. 87, 204104 (2005).
Cox, B. & Beard, P. C. Photoacoustic tomography with a single detector in a reverberant cavity. J. Acoustical Soc. Am. 125, 1426–1436 (2009).
Wang, L. V. Photo acoustic tomography. Scholarpedia 9, 10278 (2014).
Beard, P. C. Biomedical photoacoustic imaging. Interface Focus 1, 602–631 (2011).
Treeby, B. & Cox, B. k-Wave: MATLAB toolbox for the simulation and reconstruction of photoacoustic wave-fields. J. Biomed. Opt. 15, 021314 (2010).
Yao, J. et al. High-speed label-free functional photoacoustic microscopy of mouse brain in action. Nat. Methods 12, 407–410 (2015).
Liao, L. D. et al. Transcranial imaging of functional cerebral hemodynamic changes in single blood vessels using in vivo photoacoustic microscopy. J. Cerebral Blood Flow Metabolism 2, 938–951 (2012).
Hai, P., Yao, J., Maslov, K., Zhou, Y. & Wang, L. V. Near-infrared optical-resolution photoacoustic microscopy. Opt. Lett. 39, 5192–5195 (2014).
Hsu, H.-C. et al. Dual-axis illumination for virtually augmenting the detection view of optical-resolution photoacoustic microscopy. J. Biomed. Opt. 23, 076001 (2018).
Georgianos, P. I., Pikilidou, M. I., Liakopoulos, V., Balaska, E. V. & Zebekakis, P. E. Arterial stiffness in end-stage renal disease—pathogenesis, clinical epidemiology, and therapeutic potentials. Hypertension Res. 41, 309–319 (2018).
London, G. M. & Guerin, A. P. Influence of arterial pulse and reflected waves on blood pressure and cardiac function. Am. Heart J. 138, S220–S224 (1999).
Yeh, C., Hu, S., Maslov, K. & Wang, L. V. Photoacoustic microscopy of blood pulse wave. J. Biomed. Opt. 17, 070504 (2012).
Fitch, R. M., Vergona, R., Sullivan, M. E. & Wang, Y.-X. Nitric oxide synthase inhibition increases aortic stiffness measured by pulse wave velocity in rats. Cardiovasc. Res. 51, 351–358 (2001).
Seki, J. Flow pulsation and network structure in mesenteric microvasculature of rats. Am. J. Physiol. Heart Circulatory Physiol. 266, H811–H821 (1994).
Miura, G. Cancer tumor imaging: catch me if you can. Nat. Chem. Biol. 10, 485 (2014).
Hai, P. et al. Label-free high-throughput detection and quantification of circulating melanoma tumor cell clusters by linear-array-based photoacoustic tomography. J. Biomed. Opt. 22, 041004 (2017).
Dean-Ben, X. L. & Razansky, D. Localization optoacoustic tomography. Light Sci. Appl. 7, 18004 (2018).
Liang, Y., Jin, L., Guan, B.-O. & Wang, L. 2 MHz multi-wavelength pulsed laser for functional photoacoustic microscopy. Opt. Lett. 42, 1452–1455 (2017).
Li, L. et al. Small near-infrared photochromic protein for photoacoustic multi-contrast imaging and detection of protein interactions in vivo. Nat. Commun. 9, 2734 (2018).
Wu, Z. et al. A microrobotic system guided by photoacoustic computed tomography for targeted navigation in intestines in vivo. Sci. Robot. 4, eaax0613 (2019).
Moderne Messmethoden der Physik Vol. 1, 2, Extended edition (Deutscher Verlag der Wissenschaften, 1960).
Laser Institute of America. American National Standard for safe use of lasers (American National Standards Institute, 2000).
Bioucas-Dias, J. M. & Figueiredo, M. A. T. A new TwIST: two-step iterative shrinkage/thresholding algorithms for image restoration. IEEE Trans. Image Processing 16, 2992–3004 (2007).
Jacobs, J. D. & Hopper-Borge, E. A. Carotid artery infusions for pharmacokinetic and pharmacodynamic analysis of taxanes in mice. J. Vis. Exp. 92, e51917 (2014).
Winkler, A. M., Maslov, K. & Wang, L. V. Noise-equivalent sensitivity of photoacoustics. J. Biomed. Opt. 18, 097003 (2013).
Thompson, R. E., Larson, D. R. & Webb, W. W. Precise nanometer localization analysis for individual fluorescent probes. Biophys. J. 82, 2775–2783 (2002).
Acknowledgements
We thank J. Ballard and C. Ma for close reading of the manuscript, Y. He and C. Yeh for technical support, and P. Hai for his image superposition codes. This work was sponsored by National Institutes of Health Grants DP1 EB016986 (NIH Director’s Pioneer Award), R01 CA186567 (NIH Director’s Transformative Research Award), R01 EB016963, U01 NS090579 (NIH BRAIN Initiative) and U01 NS099717 (NIH BRAIN Initiative).
Author information
Authors and Affiliations
Contributions
Y.L. and L.L. designed the study. Y.L., L.L. and K.M. built the imaging system. L.L. and Y.L. planned the experiments. Y.L., L.L., E.B. and J.Y. performed the experiments. J.S. and L.W. developed the data acquisition program. L.Z., Y.L. and L.L. developed the reconstruction algorithm. Y.L., L.L., L.Z., J.L., P.H. and J.Y. analysed the data. L.V.W. conceived the concept and supervised the project. All authors contributed to writing the manuscript.
Corresponding author
Ethics declarations
Competing interests
L.V.W. and K.M. have financial interests in Microphotoacoustics, Inc., CalPACT, LLC and Union Photoacoustic Technologies, Ltd, which did not support this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–10 and Supplementary Notes 1–4.
Supplementary Video 1
Demonstration of PATER’s imaging mechanism.
Supplementary Video 2
Quantification of the spatial resolution of snapshot wide-field imaging by PATER.
Supplementary Video 3
Snapshot wide-field imaging by PATER of blood flow behind biological tissue.
Supplementary Video 4
Snapshot wide-field functional PATER imaging of haemoglobin responses in a mouse brain to front-paw stimulations in vivo.
Supplementary Video 5
Visualization of blood pulse wave propagation in the middle cerebral arteries.
Supplementary Video 6
Snapshot wide-field tracking of MTCs in a tube using PATER at 660 nm light illumination.
Supplementary Video 7
Snapshot wide-field tracking of MTCs in a mouse brain in vivo using PATER at 660 nm light illumination.
Supplementary Video 8
Close-up slow-motion video of snapshot wide-field tracking of MTCs as shown in Supplementary Video 6.
Supplementary Video 9
Buildup of MTC localization map. The positions of migrating MTCs in the blood vessels were tracked throughout the video from Supplementary Video 6 and superimposed.
Rights and permissions
About this article
Cite this article
Li, Y., Li, L., Zhu, L. et al. Snapshot photoacoustic topography through an ergodic relay for high-throughput imaging of optical absorption. Nat. Photonics 14, 164–170 (2020). https://doi.org/10.1038/s41566-019-0576-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41566-019-0576-2
This article is cited by
-
Free-moving-state microscopic imaging of cerebral oxygenation and hemodynamics with a photoacoustic fiberscope
Light: Science & Applications (2024)
-
A mathematical model for simulating photoacoustic signal generation and propagation in biological tissues
Optical and Quantum Electronics (2024)
-
Functional photoacoustic imaging: from nano- and micro- to macro-scale
Nano Convergence (2023)
-
Fast capturing of deep blood flow
Nature Biomedical Engineering (2023)
-
Ultrafast longitudinal imaging of haemodynamics via single-shot volumetric photoacoustic tomography with a single-element detector
Nature Biomedical Engineering (2023)