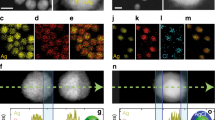
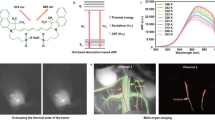
Abstract
The optically transparent biological window in the near-infrared (NIR) spectral range allows deep-tissue excitation and the detection of fluorescence signals1,2. Spectrum-domain discrimination of NIR contrast agents via an upconversion or downshifting scheme requires sufficient (anti-) Stokes shift to separate excitation and fluorescence emission. Here, we report a time-domain (τ) scheme in which about 5,000 ytterbium signal transducers are condensed within an optically inert and biocompatible CaF2 shell (2.3 nm), which forms a 14.5 nm τ-dot. Because of the long-lived and spectrally narrowly defined excited state of pure ytterbium ions, the NIR τ-dot can convert the NIR pulsed excitation into long-decaying luminescence with an efficiency approaching 100%. Within a safe injection dosage of 13 μg g−1, an excitation power density of 1.1 mW cm−2 was sufficient to image organs with a signal-to-noise ratio of >9. The high brightness of τ-dots further allows long-term in vivo passive targeting and dynamic tracking in a tumour-bearing mouse model.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data that support the plots within this paper and other findings of this study are available from the corresponding authors upon reasonable request.
Change history
19 June 2019
In the version of this Letter originally published online, in the sentence beginning “Here, we report a time-domain (τ) scheme”, ‘>5,000 ytterbium signal transducers’ should have read ‘about 5,000 ytterbium signal transducers’. And in the graph labelled ‘Downshifting’ in Fig. 1d the red peak was labelled ‘Em.’; instead it should have been labelled ‘Ex.’ These errors have now been corrected in all versions.
09 June 2021
A Correction to this paper has been published: https://doi.org/10.1038/s41566-021-00834-7
References
Frangioni, J. V. In vivo near-infrared fluorescence imaging. Curr. Opin. Chem. Biol. 7, 626–634 (2003).
Smith, A. M., Mancini, M. C. & Nie, S. Bioimaging: second window for in vivo imaging. Nat. Nanotechnol. 4, 710–711 (2009).
Nguyen, Q. T. & Tsien, R. Y. Fluorescence-guided surgery with live molecular navigation—a new cutting edge. Nat. Rev. Cancer 13, 653–662 (2013).
Vahrmeijer, A. L., Hutteman, M., van der Vorst, J. R., van de Velde, C. J. H. & Frangioni, J. V. Image-guided cancer surgery using near-infrared fluorescence. Nat. Rev. Clin. Oncol. 10, 507–518 (2013).
Medintz, I. L., Uyeda, H. T., Goldman, E. R. & Mattoussi, H. Quantum dot bioconjugates for imaging, labelling and sensing. Nat. Mater. 4, 435–446 (2005).
Alivisatos, A. P. Semiconductor clusters, nanocrystals and quantum dots. Science 271, 933–937 (1996).
Resch-Genger, U., Grabolle, M., Cavaliere-Jaricot, S., Nitschke, R. & Nann, T. Quantum dots versus organic dyes as fluorescent labels. Nat. Methods 5, 763–775 (2008).
Bünzli, J. G. & Eliseeva, S. V. Lanthanide NIR luminescence for telecommunications, bioanalyses and solar energy conversion. J. Rare Earths 28, 824–842 (2010).
Zhou, B., Shi, B., Jin, D. & Liu, X. Controlling upconversion nanocrystals for emerging applications. Nat. Nanotechnol. 10, 924–936 (2015).
Auzel, F. Upconversion and anti-Stokes processes with f and d ions in solids. Chem. Rev. 104, 139–174 (2004).
Zhou, J., Liu, Z. & Li, F. Upconversion nanophosphors for small-animal imaging. Chem. Soc. Rev. 41, 1323–1349 (2012).
Naczynski, D. J. et al. Rare-earth-doped biological composites as in vivo shortwave infrared reporters. Nat. Commun. 4, 2199 (2013).
Stouwdam, J. W. & Van Veggel, F. C. J. M. Near-infrared emission of redispersible Er3+, Nd3+ and Ho3+ doped LaF3 nanoparticles. Nano Lett. 2, 733–737 (2002).
Boyer, J. C. & Van Veggel, F. C. J. M. Absolute quantum yield measurements of colloidal NaYF4: Er3+, Yb3+ upconverting nanoparticles. Nanoscale 2, 1417–1419 (2010).
Wisser, M. D. et al. Enhancing quantum yield via local symmetry distortion in lanthanide-based upconverting nanoparticles. ACS Photonics 3, 1523–1530 (2016).
Zhong, Y. et al. Boosting the down-shifting luminescence of rare-earth nanocrystals for biological imaging beyond 1,500 nm. Nat. Commun. 8, 737 (2017).
Wang, F., Wang, J. & Liu, X. Direct evidence of a surface quenching effect on size-dependent luminescence of upconversion nanoparticles. Angew. Chem. Int. Ed. 49, 7456–7460 (2010).
Wen, S. et al. Advances in highly doped upconversion nanoparticles. Nat. Commun. 9, 2415 (2018).
Del Rosal, B. et al. Overcoming autofluorescence: long-lifetime infrared nanoparticles for time-gated in vivo imaging. Adv. Mater. 28, 10188–10193 (2016).
Zheng, X. et al. High-contrast visualization of upconversion luminescence in mice using time-gating approach. Anal. Chem. 88, 3449–3454 (2016).
Kobayashi, H., Ogawa, M., Alford, R., Choyke, P. L. & Urano, Y. New strategies for fluorescent probe design in medical diagnostic imaging. Chem. Rev. 110, 2620–2640 (2010).
Wang, Y. et al. Rare-earth nanoparticles with enhanced upconversion emission and suppressed rare-earth-ion leakage. Chem. Eur. J. 18, 5558–5564 (2012).
Ma, C. et al. Optimal sensitizer concentration in single upconversion nanocrystals. Nano Lett. 17, 2858–2864 (2017).
Prosposito, P. et al. Femtosecond dynamics of IR molecules in hybrid materials. J. Lumin. 94-95, 641–644 (2001).
Prosposito, P. et al. IR-luminescent molecules in hybrid materials. J. Solgel Sci. Technol. 26, 909–913 (2003).
Casalboni, M., De Matteis, F., Prosposito, P., Quatela, A. & Sarcinelli, F. Fluorescence efficiency of four infrared polymethine dyes. Chem. Phys. Lett. 373, 372–378 (2003).
Tao, Z. et al. Biological imaging using nanoparticles of small organic molecules with fluorescence emission at wavelengths longer than 1000 nm. Angew. Chem. Int. Ed. 52, 13002–13006 (2013).
Semonin, O. E. et al. Absolute photoluminescence quantum yields of IR-26 dye, PbS and PbSe quantum dots. J. Phys. Chem. Lett. 1, 2445–2450 (2010).
Greben, M., Fucikova, A. & Valenta, J. Photoluminescence quantum yield of PbS nanocrystals in colloidal suspensions. J. Appl. Phys. 117, 144306 (2015).
Hinds, S. et al. NIR-emitting colloidal quantum dots having 26% luminescence quantum yield in buffer solution. J. Am. Chem. Soc. 129, 7218–7219 (2007).
Kisler, K. et al. In vivo imaging and analysis of cerebrovascular hemodynamic responses and tissue oxygenation in the mouse brain. Nat. Protoc. 13, 1377–1402 (2018).
Lu, Y. et al. Tunable lifetime multiplexing using luminescent nanocrystals. Nat. Photon. 8, 32–36 (2013).
Fan, Y. et al. Lifetime-engineered NIR-II nanoparticles unlock multiplexed in vivo imaging. Nat. Nanotechnol. 13, 941–946 (2018).
Ortgies, D. H. et al. Lifetime-encoded infrared-emitting nanoparticles for in vivo multiplexed imaging. ACS Nano 12, 4362–4368 (2018).
Liu, Q., Feng, W., Yang, T., Yi, T. & Li, F. Upconversion luminescence imaging of cells and small animals. Nat. Protoc. 8, 2033–2044 (2013).
Li, Y. et al. Core–shell–shell NaYbF4:Tm@CaF2@NaDyF4 nanocomposites for upconversion/T2-weighted MRI/computed tomography lymphatic imaging. ACS Appl. Mater. Inter. 8, 19208–19216 (2016).
Acknowledgements
This project was primarily supported by the National Key R&D Program of China (2017YFA0205100), the National Natural Science Foundation of China (21527801, 21722101, 61729501 and 51720105015), the Strategic Seeding Fund of the UTS Institute for Biomedical Materials and Devices (IBMD) and the Australian Research Council (ARC) Future Fellowship Scheme (to D.J., FT 130100517). The authors thank X. Chen, D. Tu and W. You for their help with lifetime measurements.
Author information
Authors and Affiliations
Contributions
W.F., D.J. and F.L. conceived the project and supervised the research. Y.Gu synthesized the nanoparticles and was primarily responsible for conducting the experiments. Z.G., F.W. and D.J. designed and modified the optical spectroscopy and imaging instruments. Y.Gu, D.J., J.Z., W.F. and F.L. analysed the results, prepared the figures and wrote the manuscript. All authors participated in discussions and editing of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
This file contains more information about the work, Supplementary Figures 1–37 and Supplementary Tables 1–4.
Rights and permissions
About this article
Cite this article
Gu, Y., Guo, Z., Yuan, W. et al. High-sensitivity imaging of time-domain near-infrared light transducer. Nat. Photonics 13, 525–531 (2019). https://doi.org/10.1038/s41566-019-0437-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41566-019-0437-z
This article is cited by
-
Near-infrared II fluorescence imaging
Nature Reviews Methods Primers (2024)
-
In vivo NIR-II fluorescence imaging for biology and medicine
Nature Photonics (2024)
-
Oxyhaemoglobin saturation NIR-IIb imaging for assessing cancer metabolism and predicting the response to immunotherapy
Nature Nanotechnology (2024)
-
Time-Resolved Imaging in Short-Wave Infrared Region
Journal of Shanghai Jiaotong University (Science) (2024)
-
Bright Tm3+-based downshifting luminescence nanoprobe operating around 1800 nm for NIR-IIb and c bioimaging
Nature Communications (2023)