Abstract
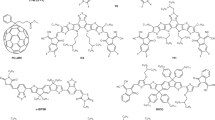
Over the past three years, a particularly exciting and active area of research within the field of organic photovoltaics has been the use of non-fullerene acceptors (NFAs). Compared with fullerene acceptors, NFAs possess significant advantages including tunability of bandgaps, energy levels, planarity and crystallinity. To date, NFA solar cells have not only achieved impressive power conversion efficiencies of ~13–14%, but have also shown excellent stability compared with traditional fullerene acceptor solar cells. This Review highlights recent progress on single-junction and tandem NFA solar cells and research directions to achieve even higher efficiencies of 15–20% using NFA-based organic photovoltaics are also proposed.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Li, G., Zhu, R. & Yang, Y. Polymer solar cells. Nat. Photon. 6, 153–161 (2012).
Graetzel, M., Janssen, R. A. J., Mitzi, D. B. & Sargent, E. H. Materials interface engineering for solution-processed photovoltaics. Nature 488, 304–312 (2012).
Heeger, A. J. 25th anniversary article: bulk heterojunction solar cells: understanding the mechanism of operation. Adv. Mater. 26, 10–27 (2014).
Brabec, C. J., Heeney, M., McCulloch, I. & Nelson, J. Influence of blend microstructure on bulk heterojunction organic photovoltaic performance. Chem. Soc. Rev. 40, 1185–1199 (2011).
Janssen, R. A. J. & Nelson, J. Factors limiting device efficiency in organic photovoltaics. Adv. Mater. 25, 1847–1858 (2013).
Chamberlain, G. A. Organic solar cells: a review. Solar Cells 8, 47–83 (1983).
Tang, C. W. Two-layer organic photovoltaic cell. Appl. Phys. Lett. 48, 183–185 (1986).
Yu, G., Gao, J., Hummelen, J. C., Wudl, F. & Heeger, A. J. Polymer photovoltaic cells—enhanced efficiencies via a network of internal donor-acceptor heterojunctions. Science 270, 1789–1791 (1995).
Halls, J. J. M. et al. Efficient photodiodes from interpenetrating polymer networks. Nature 376, 498–500 (1995).
Blom, P. W. M., Mihailetchi, V. D., Koster, L. J. A. & Markov, D. E. Device physics of polymer: fullerene bulk heterojunction solar cells. Adv. Mater. 19, 1551–1566 (2007).
Stoltzfus, D. M. et al. Charge generation pathways in organic solar cells: assessing the contribution from the electron acceptor. Chem. Rev. 116, 12920–12955 (2016).
Hawks, S. A. et al. Relating recombination, density of states, and device performance in an efficient polymer:fullerene organic solar cell blend. Adv. Energy Mater. 3, 1201–1209 (2013).
Dennler, G., Scharber, M. C. & Brabec, C. J. Polymer-fullerene bulk-heterojunction solar cells. Adv. Mater. 21, 1323–1338 (2009).
Chen, Y., Wan, X. & Long, G. High performance photovoltaic applications using solution-processed small molecules. Acc. Chem. Res. 46, 2645–2655 (2013).
Yao, H. et al. Molecular design of benzodithiophene-based organic photovoltaic materials. Chem. Rev. 116, 7397–7457 (2016).
Chen, H.-Y. et al. Polymer solar cells with enhanced open-circuit voltage and efficiency. Nat. Photon. 3, 649–653 (2009).
Liang, Y. et al. For the bright future—bulk heterojunction polymer solar cells with power conversion efficiency of 7.4%. Adv. Mater. 22, E135–E138 (2010).
Zhou, H. et al. Development of fluorinated benzothiadiazole as a structural unit for a polymer solar cell of 7% efficiency. Angew. Chem. Int. Ed. 50, 2995–2998 (2011).
Zhou, J. et al. Small molecules based on benzo[1,2-b:4,5-b’]dithiophene unit for high-performance solution processed organic solar cells. J. Am. Chem. Soc. 134, 16345–16351 (2012).
He, Y. J., Chen, H. Y., Hou, J. H. & Li, Y. F. Indene-C60 bisadduct: a new acceptor for high-performance polymer solar cells. J. Am. Chem. Soc. 132, 1377–1382 (2010).
Chen, W. & Zhang, Q. Recent progress in non-fullerene small molecule acceptors in organic solar cells (OSCs). J. Mater. Chem. C 5, 1275–1302 (2017).
Khlyabich, P. P., Burkhart, B. & Thompson, B. C. Efficient ternary blend bulk heterojunction solar cells with tunable open-circuit voltage. J. Am. Chem. Soc. 133, 14534–14537 (2011).
Yang, L., Zhou, H., Price, S. C. & You, W. Parallel-like bulk heterojunction polymer solar cells. J. Am. Chem. Soc. 134, 5432–5435 (2012).
Yang, Y. et al. High-performance multiple-donor bulk heterojunction solar cells. Nat. Photon. 9, 190–198 (2015).
Lu, L., Kelly, M. A., You, W. & Yu, L. Status and prospects for ternary organic photovoltaics. Nat. Photon. 9, 491–500 (2015).
Ma, W., Yang, C., Gong, X., Lee, K. & Heeger, A. J. Thermally stable, efficient polymer solar cells with nanoscale control of the interpenetrating network morphology. Adv. Funct. Mater. 15, 1617–1622 (2005).
Li, G. et al. High-efficiency solution processable polymer photovoltaic cells by self-organization of polymer blends. Nat. Mater. 4, 864–868 (2005).
Peet, J. et al. Efficiency enhancement in low-bandgap polymer solar cells by processing with alkane dithiols. Nat. Mater. 6, 497–500 (2007).
Huang, F., Wu, H. B. & Cao, Y. Water/alcohol soluble conjugated polymers as highly efficient electron transporting/injection layer in optoelectronic devices. Chem. Soc. Rev. 39, 2500–2521 (2010).
He, Z. et al. Enhanced power-conversion efficiency in polymer solar cells using an inverted device structure. Nat. Photon. 6, 591–595 (2012).
Li, G., Chu, C. W., Shrotriya, V., Huang, J. & Yang, Y. Efficient inverted polymer solar cells. Appl. Phys. Lett. 88, 253503 (2006).
Wang, K., Liu, C., Meng, T., Yi, C. & Gong, X. Inverted organic photovoltaic cells. Chem. Soc. Rev. 45, 2937–2975 (2016).
Kim, J. Y. et al. Efficient tandem polymer solar cells fabricated by all-solution processing. Science 317, 222–225 (2007).
You, J. B., Dou, L. T., Hong, Z. R., Li, G. & Yang, Y. Recent trends in polymer tandem solar cells research. Prog. Polym. Sci. 38, 1909–1928 (2013).
Zhao, J. et al. Efficient organic solar cells processed from hydrocarbon solvents. Nat. Energy 1, 15027 (2016).
Zhan, X. et al. A high-mobility electron-transport polymer with broad absorption and its use in field-effect transistors and all-polymer solar cells. J. Am. Chem. Soc. 129, 7246–7247 (2007).
Few, S., Frost, J. M., Kirkpatrick, J. & Nelson, J. Influence of chemical structure on the charge transfer state spectrum of a polymer:fullerene complex. J. Phys. Chem. C 118, 8253–8261 (2014).
Jorgensen, M. et al. Stability of polymer solar cells. Adv. Mater. 24, 580–612 (2012).
Cheng, P. & Zhan, X. Stability of organic solar cells: challenges and strategies. Chem. Soc. Rev. 45, 2544–2582 (2016).
Polman, A., Knight, M., Garnett, E. C., Ehrler, B. & Sinke, W. C. Photovoltaic materials: present efficiencies and future challenges. Science 352, aad4424 (2016).
Dang, M. T., Hirsch, L. & Wantz, G. P3HT:PCBM, best seller in polymer photovoltaic research. Adv. Mater. 23, 3597–3602 (2011).
Guo, X., Facchetti, A. & Marks, T. J. Imide- and amide-functionalized polymer semiconductors. Chem. Rev. 114, 8943–9021 (2014).
Jiang, W., Li, Y. & Wang, Z. Tailor-made rylene arrays for high performance n-channel semiconductors. Acc. Chem. Res. 47, 3135–3147 (2014).
Nielsen, C. B., Holliday, S., Chen, H.-Y., Cryer, S. J. & McCulloch, I. Non-fullerene electron acceptors for use in organic solar cells. Acc. Chem. Res. 48, 2803–2812 (2015).
Kang, H. et al. From fullerene–polymer to all-polymer solar cells: the importance of molecular packing, orientation, and morphology control. Acc. Chem. Res. 49, 2424–2434 (2016).
Diao, Y. et al. Flow-enhanced solution printing of all-polymer solar cells. Nat. Commun. 6, 7955 (2015).
Song, C. J., Wang, E. J., Dong, B. H. & Wang, S. M. Non-fullerene organic small molecule acceptor materials. Prog. Chem. 27, 1754–1763 (2015).
Liu, Z., Wu, Y., Zhang, Q. & Gao, X. Non-fullerene small molecule acceptors based on perylene diimides. J. Mater. Chem. A 4, 17604–17622 (2016).
Fernandez-Lazaro, F., Zink-Lorre, N. & Sastre-Santos, A. Perylenediimides as non-fullerene acceptors in bulk-heterojunction solar cells (BHJSCs). J. Mater. Chem. A 4, 9336–9346 (2016).
Jin, R., Wang, F., Guan, R., Zheng, X. & Zhang, T. Design of perylene-diimides-based small-molecules semiconductors for organic solar cells. Mol. Phys. 115, 1591–1597 (2017).
Zhong, Y. et al. Efficient organic solar cells with helical perylene diimide electron acceptors. J. Am. Chem. Soc. 136, 15215–15221 (2014).
Chen, W. et al. A perylene diimide (PDI)-based small molecule with tetrahedral configuration as a non-fullerene acceptor for organic solar cells. J. Mater. Chem. C 3, 4698–4705 (2015).
Yan, H. et al. A high-mobility electron-transporting polymer for printed transistors. Nature 457, 679–686 (2009).
Earmme, T., Hwang, Y.-J., Murari, N. M., Subramaniyan, S. & Jenekhe, S. A. All-polymer solar cells with 3.3% efficiency based on naphthalene diimide-selenophene copolymer acceptor. J. Am. Chem. Soc. 135, 14960–14963 (2013).
Jung, J. W. et al. Fluoro-substituted n-type conjugated polymers for additive-free all-polymer bulk heterojunction solar cells with high power conversion efficiency of 6.71%. Adv. Mater. 27, 3310–3317 (2015).
Mori, D., Benten, H., Ohkita, H., Ito, S. & Miyake, K. Polymer/polymer blend solar cells improved by using high-molecular-weight fluorene-based copolymer as electron acceptor. ACS Appl. Mater. Interfaces 4, (3325–3329 (2012).
Bloking, J. T. et al. Solution-processed organic solar cells with power conversion efficiencies of 2.5% using benzothiadiazole/imide-based acceptors. Chem. Mater. 23, 5484–5490 (2011).
Cnops, K. et al. 8.4% efficient fullerene-free organic solar cells exploiting long-range exciton energy transfer. Nat. Commun. 5, 3406 (2014).
Brunetti, F. G., Gong, X., Tong, M., Heeger, A. J. & Wudl, F. Strain and Hückel aromaticity: driving forces for a promising new generation of electron acceptors in organic electronics. Angew. Chem. Int. Ed. 49, 532–536 (2010).
Yan, Q., Zhou, Y., Zheng, Y.-Q., Pei, J. & Zhao, D. Toward rational design of organic electron acceptor for photovoltaics: a study based on perylenediimide derivatives. Chem. Sci. 4, 4389–4394 (2013).
Zhang, X. et al. A potential perylene diimide dimer-based acceptor material for highly efficient solution-processed non-fullerene organic solar cells with 4.03% efficiency. Adv. Mater. 25, 5791–5797 (2013).
Liu, Y. et al. A tetraphenylethylene core-based 3D structure small molecular acceptor enabling efficient non-fullerene organic solar cells. Adv. Mater. 27, 1015–1020 (2015).
Zhong, Y. et al. Molecular helices as electron acceptors in high-performance bulk heterojunction solar cells. Nat. Commun. 6, 8242 (2015).
Liu, J. et al. Fast charge separation in a non-fullerene organic solar cell with a small driving force. Nat. Energy 1, 16089 (2016).
Duan, Y. et al. Pronounced effects of a triazine core on photovoltaic performance–efficient organic solar cells enabled by a pdi trimer-based small molecular acceptor. Adv. Mater. 29, 1605115 (2017).
Sisto, T. J. et al. Long, atomically precise donor–acceptor cove-edge nanoribbons as electron acceptors. J. Am. Chem. Soc. 139, 5648–5651 (2017).
Li, Y. F. Molecular design of photovoltaic materials for polymer solar cells: Toward suitable electronic energy levels and broad absorption. Acc. Chem. Res. 45, 723–733 (2012).
Lin, Y. et al. An electron acceptor challenging fullerenes for efficient polymer solar cells. Adv. Mater. 27, 1170–1174 (2015).
Chen, C.-P., Chan, S.-H., Chao, T.-C., Ting, C. & Ko, B.-T. Low-bandgap poly(thiophene-phenylene-thiophene) derivatives with broaden absorption spectra for use in high-performance bulk-heterojunction polymer solar cells. J. Am. Chem. Soc. 130, 12828–12833 (2008).
Wong, K.-T. et al. Syntheses and structures of novel heteroarene-fused coplanar π-conjugated chromophores. Org. Lett. 8, 5033–5036 (2006).
Zhang, Y. et al. Indacenodithiophene and quinoxaline-based conjugated polymers for highly efficient polymer solar cells. Chem. Mater. 23, 2289–2291 (2011).
He, G. et al. Efficient small molecule bulk heterojunction solar cells with high fill factors via introduction of [small pi]-stacking moieties as end group. J. Mater. Chem. A 1, 1801–1809 (2013).
Bin, H. et al. 11.4% Efficiency non-fullerene polymer solar cells with trialkylsilyl substituted 2D-conjugated polymer as donor. Nat. Commun. 7, 13651 (2016).
Lin, Y. et al. High-performance electron acceptor with thienyl side chains for organic photovoltaics. J. Am. Chem. Soc. 138, 4955–4961 (2016).
Yang, Y. et al. Side-chain isomerization on n-type organic semiconductor ITIC acceptor make 11.77% high efficiency polymer solar cells. J. Am. Chem. Soc. 138, 15011–15018 (2016).
Li, Z. et al. Donor polymer design enables efficient non-fullerene organic solar cells. Nat. Commun. 7, 13094 (2016).
Yao, H. et al. Achieving highly efficient nonfullerene organic solar cells with improved intermolecular interaction and open-circuit voltage. Adv. Mater. 29, 1700254 (2017).
Lin, Y. et al. Mapping polymer donors toward high-efficiency fullerene free organic solar cells. Adv. Mater. 29, 1604155 (2017).
Holliday, S. et al. High-efficiency and air-stable P3HT-based polymer solar cells with a new non-fullerene acceptor. Nat. Commun. 7, 11585 (2016).
Baran, D. et al. Reducing the efficiency-stability-cost gap of organic photovoltaics with highly efficient and stable small molecule acceptor ternary solar cells. Nat. Mater. 16, 363–369 (2017).
Guo, Y. et al. A Vinylene-bridged perylenediimide-based polymeric acceptor enabling efficient all-polymer solar cells processed under ambient conditions. Adv. Mater. 28, 8483–8489 (2016).
Wang, W. et al. Fused hexacyclic nonfullerene acceptor with strong near-infrared absorption for semitransparent organic solar cells with 9.77% efficiency. Adv. Mater. 29, 1701308 (2017).
Yao, H. et al. Design and synthesis of a low bandgap small molecule acceptor for efficient polymer solar cells. Adv. Mater. 28, 8283–8287 (2016).
Lin, Y. et al. High-performance fullerene-free polymer solar cells with 6.31% efficiency. Energy Environ. Sci. 8, 610–616 (2015).
Yao, H. et al. Design, synthesis, and photovoltaic characterization of a small molecular acceptor with an ultra-narrow band gap. Angew. Chem. Int. Ed. 56, 3045–3049 (2017).
Zhao, W. et al. Molecular optimization enables over 13% efficiency in organic solar cells. J. Am. Chem. Soc. 139, 7148–7151 (2017).
Gao, L. et al. All-polymer solar cells based on absorption-complementary polymer donor and acceptor with high power conversion efficiency of 8.27%. Adv. Mater. 28, 1884–1890 (2016).
Melzer, C., Koop, E. J., Mihailetchi, V. D. & Blom, P. W. M. Hole transport in poly(phenylene vinylene)/methanofullerene bulk-heterojunction solar cells. Adv. Funct. Mater. 14, 865–870 (2004).
Wu, Q., Zhao, D., Schneider, A. M., Chen, W. & Yu, L. Covalently bound clusters of alpha-substituted pdi—rival electron acceptors to fullerene for organic solar cells. J. Am. Chem. Soc. 138, 7248–7251 (2016).
Azzopardi, B. et al. Economic assessment of solar electricity production from organic-based photovoltaic modules in a domestic environment. Energy Environ. Sci. 4, 3741–3753 (2011).
Li, S. et al. A spirobifluorene and diketopyrrolopyrrole moieties based non-fullerene acceptor for efficient and thermally stable polymer solar cells with high open-circuit voltage. Energy Environ. Sci. 9, 604–610 (2016).
Holliday, S. et al. A rhodanine flanked nonfullerene acceptor for solution-processed organic photovoltaics. J. Am. Chem. Soc. 137, 898–904 (2015).
Savagatrup, S. et al. Mechanical degradation and stability of organic solar cells: molecular and microstructural determinants. Energy Environ. Sci. 8, 55–80 (2015).
Kim, T. et al. Flexible, highly efficient all-polymer solar cells. Nat. Commun. 6, 8547 (2015).
Brédas, J.-L., Beljonne, D., Coropceanu, V. & Cornil, J. Charge-transfer and energy-transfer processes in π-conjugated oligomers and polymers: a molecular picture. Chem. Rev. 104, 4971–5004 (2004).
Cheng, P. et al. Realizing small energy loss of 0.55 eV, high open-circuit voltage >1 V and high efficiency >10% in fullerene-free polymer solar cells via energy driver. Adv. Mater. 29, 1605216 (2017).
Yao, J. et al. Quantifying losses in open-circuit voltage in solution-processable solar cells. Phys. Rev. Appl. 4, 014020 (2015).
Tuladhar, S. M. et al. Low open-circuit voltage loss in solution-processed small-molecule organic solar cells. ACS Energy Lett. 1, 302–308 (2016).
Vandewal, K. et al. Quantification of quantum efficiency and energy losses in low bandgap polymer:fullerene solar cells with high open-circuit voltage. Adv. Funct. Mater. 22, 3480–3490 (2012).
Baran, D. et al. Reduced voltage losses yield 10% and >1V fullerene free organic solar cells. Energy Environ. Sci. 9, 3783–3793 (2016).
Chen, S. et al. A wide-bandgap donor polymer for highly efficient non-fullerene organic solar cells with a small voltage loss. J. Am. Chem. Soc. 139, 6298–6301 (2017).
Liu, D. et al. Molecular design of a wide-band-gap conjugated polymer for efficient fullerene-free polymer solar cells. Energy Environ. Sci. 10, 546–551 (2017).
Ameri, T., Dennler, G., Lungenschmied, C. & Brabec, C. J. Organic tandem solar cells: a review. Energy Environ. Sci. 2, 347–363 (2009).
Shockley, W. & Queisser, H. J. Detailed balance limit of efficiency of p‐n junction solar cells. J. Appl. Phys. 32, 510–519 (1961).
Green, M. A. et al. Solar cell efficiency tables (version 50). Prog. Photovoltaics 25, 668–676 (2017).
Yakimov, A. & Forrest, S. R. High photovoltage multiple-heterojunction organic solar cells incorporating interfacial metallic nanoclusters. Appl. Phys. Lett. 80, 1667–1669 (2002).
Ameri, T., Li, N. & Brabec, C. J. Highly efficient organic tandem solar cells: follow up review. Energy Environ. Sci. 6, 2390–2413 (2013).
Hadipour, A. et al. Solution-processed organic tandem solar cells. Adv. Funct. Mater. 16, 1897–1903 (2006).
Dou, L. et al. Tandem polymer solar cells featuring a spectrally matched low-bandgap polymer. Nat. Photon. 6, 180–185 (2012).
You, J. et al. A polymer tandem solar cell with 10.6% power conversion efficiency. Nat. Commun. 4, 1446 (2013).
Andersen, T. R. et al. Scalable, ambient atmosphere roll-to-roll manufacture of encapsulated large area, flexible organic tandem solar cell modules. Energy Environ. Sci. 7, 2925–2933 (2014).
Spyropoulos, G. D. et al. Flexible organic tandem solar modules with 6% efficiency: combining roll-to-roll compatible processing with high geometric fill factors. Energy Environ. Sci. 7, 3284–3290 (2014).
Zuo, L. et al. Design of a versatile interconnecting layer for highly efficient series-connected polymer tandem solar cells. Energy Environ. Sci. 8, 1712–1718 (2015).
Zhang, K. et al. High-performance polymer tandem solar cells employing a new n-type conjugated polymer as an interconnecting layer. Adv. Mater. 28, 4817–4823 (2016).
Li, M. et al. Solution-processed organic tandem solar cells with power conversion efficiencies >12%. Nat. Photon. 11, 85–90 (2017).
You, J. et al. 10.2% power conversion efficiency polymer tandem solar cells consisting of two identical sub-cells. Adv. Mater. 25, 3973–3978 (2013).
Zhou, H. et al. Polymer homo-tandem solar cells with best efficiency of 11.3%. Adv. Mater. 27, 1767–1773 (2015).
Li, W., Furlan, A., Hendriks, K. H., Wienk, M. M. & Janssen, R. A. J. Efficient tandem and triple-junction polymer solar cells. J. Am. Chem. Soc. 135, 5529–5532 (2013).
Chen, C.-C. et al. An efficient triple-junction polymer solar cell having a power conversion efficiency exceeding 11%. Adv. Mater. 26, 5670–5677 (2014).
Yusoff, A. R. b. M. et al. A high efficiency solution processed polymer inverted triple-junction solar cell exhibiting a power conversion efficiency of 11.83%. Energy Environ. Sci. 8, 303–316 (2015).
Liu, W. et al. Nonfullerene tandem organic solar cells with high open-circuit voltage of 1.97 V. Adv. Mater. 28, 9729–9734 (2016).
Qin, Y. et al. Achieving 12.8% efficiency by simultaneously improving open-circuit voltage and short-circuit current density in tandem organic solar cells. Adv. Mater. 29, 1606340 (2017).
Cui, Y. et al. Fine tuned photoactive and interconnection layers for achieving over 13% efficiency in a fullerene-free tandem organic solar cell. J. Am. Chem. Soc. 139, 7302–7309 (2017).
Yuan, J. et al. High efficiency all-polymer tandem solar cells. Sci. Rep. 6, 26459 (2016).
Chen, S. et al. An all-solution processed recombination layer with mild post-treatment enabling efficient homo-tandem non-fullerene organic solar cells. Adv. Mater. 29, 1604231 (2017).
Lu, L. et al. Recent advances in bulk heterojunction polymer solar cells. Chem. Rev. 115, 12666–12731 (2015).
Dennler, G. et al. Design rules for donors in bulk-heterojunction tandem solar cells—towards 15% energy-conversion efficiency. Adv. Mater. 20, 579–583 (2008).
Li, G., Chang, W.-H. & Yang, Y. Low-bandgap conjugated polymers enabling solution-processable tandem solar cells. Nat. Rev. Mater. 2, 17043 (2017).
Acknowledgements
Y.Y. acknowledges the Air Force Office of Scientific Research (AFOSR) (FA2386-15-1-4108), Office of Naval Research (ONR) (N00014-14-1-0648), National Science Foundation (NSF) (ECCS-1509955) and UC-Solar Program (MRPI 328368) for financial support. X.Z. acknowledges the National Science Foundation China (NSFC) (51761165023, 21734001) for financial support. G.L. acknowledges the Project of Strategic Importance provided by The Hong Kong Polytechnic University (1-ZE29) for financial support. All authors acknowledge N. De Marco for help with English language editing.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Abbreviations, chemical structures and calculations.
Rights and permissions
About this article
Cite this article
Cheng, P., Li, G., Zhan, X. et al. Next-generation organic photovoltaics based on non-fullerene acceptors. Nature Photon 12, 131–142 (2018). https://doi.org/10.1038/s41566-018-0104-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41566-018-0104-9
This article is cited by
-
Physical insights into non-fullerene organic photovoltaics
Nature Reviews Physics (2024)
-
The Photocharging Effect and Part Electronic Structure Changes of Organic Semiconductors in Photoelectrochemical Water Splitting
Catalysis Letters (2024)
-
A new nonfullerene acceptor with an extended π conjugation core enables ternary organic solar cells approaching 19% efficiency
Nano Research (2024)
-
Optimization of Non-fullerene Organic Photovoltaics Through Interface Engineering with Graphene Oxide: A Numerical Simulation
Journal of Electronic Materials (2024)
-
Enhanced photovoltaic performance of A–D–A’–D–A type non-fused ring electron acceptors via side chain engineering
Science China Materials (2024)