Abstract
Type VI CRISPR systems protect against phage infection using the RNA-guided nuclease Cas13 to recognize viral messenger RNA. Upon target recognition, Cas13 cleaves phage and host transcripts non-specifically, leading to cell dormancy that is incompatible with phage propagation. However, whether and how infected cells recover from dormancy is unclear. Here we show that type VI CRISPR and DNA-cleaving restriction-modification (RM) systems frequently co-occur and synergize to clear phage infections and resuscitate cells. In the natural type VI CRISPR host Listeria seeligeri, we show that RM cleaves the phage genome, thus removing the source of phage transcripts and enabling cells to recover from Cas13-induced cellular dormancy. We find that phage infections are neutralized more effectively when Cas13 and RM systems operate together. Our work reveals that type VI CRISPR immunity is cell-autonomous and non-abortive when paired with RM, and hints at other synergistic roles for the diverse host-directed immune systems in bacteria.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All L. seeligeri genome sequences reported here have been uploaded to NCBI through Genbank under BioProject accession number PRJNA901958. RM loci are publically available through the REBASE database (rebase.neb.com). Lists of strains, plasmids and oligonucleotides used in this study are available in Supplementary Table 2. Further information and requests for resources and reagents should be directed to and will be fulfilled by the corresponding author. Source data are provided with this paper.
References
Tal, N. & Sorek, R. SnapShot: bacterial immunity. Cell 185, 578-578.e1 (2022).
Rostol, J. T. & Marraffini, L. (Ph)ighting phages: how bacteria resist their parasites. Cell Host Microbe 25, 184–194 (2019).
Barrangou, R. et al. CRISPR provides acquired resistance against viruses in prokaryotes. Science 315, 1709–1712 (2007).
Marraffini, L. A. & Sontheimer, E. J. CRISPR interference limits horizontal gene transfer in staphylococci by targeting DNA. Science 322, 1843–1845 (2008).
Watanabe, T. et al. CRISPR regulation of intraspecies diversification by limiting IS transposition and intercellular recombination. Genome Biol. Evol. 5, 1099–1114 (2013).
Abudayyeh, O. O. et al. C2c2 is a single-component programmable RNA-guided RNA-targeting CRISPR effector. Science 353, aaf5573 (2016).
Meeske, A. J., Nakandakari-Higa, S. & Marraffini, L. A. Cas13-induced cellular dormancy prevents the rise of CRISPR-resistant bacteriophage. Nature 570, 241–245 (2019).
Mendoza, S. D. et al. A bacteriophage nucleus-like compartment shields DNA from CRISPR nucleases. Nature 577, 244–248 (2020).
Adler, B. A. et al. Broad-spectrum CRISPR-Cas13a enables efficient phage genome editing. Nat. Microbiol. 7, 1967–1979 (2022).
Meeske, A. J. et al. A phage-encoded anti-CRISPR enables complete evasion of type VI-A CRISPR-Cas immunity. Science 369, 54–59 (2020).
Oliveira, P. H., Touchon, M. & Rocha, E. P. The interplay of restriction-modification systems with mobile genetic elements and their prokaryotic hosts. Nucleic Acids Res. 42, 10618–10631 (2014).
Bickle, T. A. & Krüger, D. Biology of DNA restriction. Microbiol. Rev. 57, 434–450 (1993).
Arber, W. Host-controlled modification of bacteriophage. Annu. Rev. Microbiol. 19, 365–378 (1965).
Dupuis, M. E., Villion, M., Magadan, A. H. & Moineau, S. CRISPR-Cas and restriction-modification systems are compatible and increase phage resistance. Nat. Commun. 4, 2087 (2013).
Maguin, P., Varble, A., Modell, J. W. & Marraffini, L. A. Cleavage of viral DNA by restriction endonucleases stimulates the type II CRISPR-Cas immune response. Mol. Cell 82, 907–919.e7 (2022).
Meeske, A. J. & Marraffini, L. A. RNA guide complementarity prevents self-targeting in type VI CRISPR systems. Mol. Cell 71, 791–801.e3 (2018).
Deutscher, M. P. Maturation and degradation of ribosomal RNA in bacteria. Prog. Mol. Biol. Transl. Sci. 85, 369–391 (2009).
Robinett, C. C. et al. In vivo localization of DNA sequences and visualization of large-scale chromatin organization using lac operator/repressor recognition. J. Cell Biol. 135, 1685–1700 (1996).
Bernheim, A. & Sorek, R. The pan-immune system of bacteria: antiviral defence as a community resource. Nat. Rev. Microbiol. 18, 113–119 (2020).
Guegler, C. K. & Laub, M. T. Shutoff of host transcription triggers a toxin-antitoxin system to cleave phage RNA and abort infection. Mol. Cell 81, 2361–2373.e9 (2021).
Fineran, P. C. et al. The phage abortive infection system, ToxIN, functions as a protein-RNA toxin-antitoxin pair. Proc. Natl Acad. Sci. USA 106, 894–899 (2009).
Makarova, K. S., Anantharaman, V., Aravind, L. & Koonin, E. V. Live virus-free or die: coupling of antivirus immunity and programmed suicide or dormancy in prokaryotes. Biol. Direct 7, 40 (2012).
Simon, R., Priefer, U. & Puhler, A. A broad host range mobilization system for in vivo genetic-engineering: transposon mutagenesis in Gram-negative bacteria. Biotechnology 1, 784–791 (1983).
Lauer, P., Chow, M. Y., Loessner, M. J., Portnoy, D. A. & Calendar, R. Construction, characterization, and use of two Listeria monocytogenes site-specific phage integration vectors. J. Bacteriol. 184, 4177–4186 (2002).
Demarre, G. et al. A new family of mobilizable suicide plasmids based on broad host range R388 plasmid (IncW) and RP4 plasmid (IncPalpha) conjugative machineries and their cognate Escherichia coli host strains. Res. Microbiol. 156, 245–255 (2005).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Payne, L. J. et al. Identification and classification of antiviral defence systems in bacteria and archaea with PADLOC reveals new system types. Nucleic Acids Res. 49, 10868–10878 (2021).
Acknowledgements
We thank all members of the Meeske lab for advice and encouragement, members of the Guo and Mitchell labs for helpful discussions, R. Calendar (UC Berkeley) for providing A118 and U153 phages, and D. Rudner (Harvard Medical School)/X. Wang (Indiana University) for providing plasmid templates pWX510 and pWX570. Support for this work came from the NIH (R35GM142460, S10OD026741) and the University of Washington Royalty Research Foundation.
Author information
Authors and Affiliations
Contributions
The project was conceived by M.C.W., A.E.R. and A.J.M. Experiments were performed by M.C.W., A.E.R. and A.J.M. Bioinformatic analysis was conducted by M.C.W., S.R.M. and A.J.M. J.L. and M.W. isolated L. seeligeri strains. Pilot microscopy experiments were performed with assistance from E.R.R. The paper was written and edited by M.C.W., A.E.R., S.R.M. and A.J.M.
Corresponding author
Ethics declarations
Competing interests
A.J.M. is a co-founder of Profluent Bio. The other authors declare no competing interests.
Peer review
Peer review information
Nature Microbiology thanks Mitchell O’Connell and the other, anonymous, reviewer(s) for their contribution to the peer review of this work
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Bioinformatic analysis of anti-phage defenses in L. seeligeri.
(a) Anti-phage defense systems detected in 62 L. seeligeri strains using PADLOC. (b) Genomic locations of CRISPR and RM systems within all 62 L. seeligeri genomes. Each genomic location was normalized to the position of the well-conserved, origin-proximal dnaA gene. (c) Percentage of 62 L. seeligeri genomes harbouring the indicated RM type. (d) Combinations of RM types observed in 62 L. seeligeri genomes. Filled blue rectangles indicate the presence of the indicated RM type. (e) Tally of RM systems per genome in Listeria strains with or without the indicated CRISPR type. Error bars denote standard error of the mean.
Extended Data Fig. 2 Both type I RM systems are expressed in L. seeligeri LS1.
(a)–(b) Diagrams of type I RM loci in the LS1 genome (methylase denoted M, specificity subunit denoted S, endonuclease denoted R, one locus also has a type IV nuclease denoted Mrr). RNA-seq read coverage at each nucleotide position of the two loci are plotted. The y-axis reflects the number of mapped reads overlapping each nucleotide position. (c) RNA-seq reads corresponding to the LS1 type VI-A CRISPR-Cas locus. (d) Table of expression values (RPKM, reads per kilobase per million mapped reads) for LS1 RM genes and cas genes, as well as essential genes rodA, ponA, rnc, sigA, and a transcript induced under Ptet control by the addition of aTc (anhydrotetracycline).
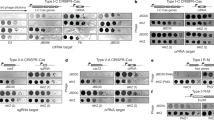
Extended Data Fig. 3 ϕLS59 is sensitive to both RM and type VI CRISPR immunity.
Plaque assay of ϕLS59 on a set of L. seeligeri strains lacking RM and type VI CRISPR immunity (∆RM, nt spc), equipped with RM immunity only (WT, nt spc), or equipped with CRISPR immunity only targeting early lytic genes (∆RM, spcE) or late lytic genes (∆RM, spcL). Serial tenfold dilutions were made from the phage stock, and 2 µL of each dilution was spotted on the indicated bacterial lawn.
Extended Data Fig. 4 Clearance of phage and recovery of phage-sensitive cells after ϕLS59 infection.
(a) PFU titers from infection cultures shown in Fig. 3c at 24 hours post-infection. PFU were enumerated on a lawn of LS1 ∆RM nt spc. NT = non-targeting spacer, spcE = spacer targeting early lytic gene, spcL = spacer targeting late lytic gene. Analysis of 3 biological replicates is shown. (b) ϕLS59 adsorption assays performed on six surviving isolates from each the infections in Fig. 3c. (c) PCR with ϕLS59-specific primers performed on six surviving isolates from each of the infections in Fig. 3c, as well as positive control PCRs using the phage stock and a confirmed ϕLS59 lysogen.
Extended Data Fig. 5 ϕLS59 tetOx60 is viable and sensitive to both RM and type VI CRISPR immunity.
Plaque assay of ϕLS59 tetOx60 on a set of L. seeligeri strains lacking RM and type VI CRISPR immunity (∆RM, nt spc), equipped with RM immunity only (WT, nt spc), or equipped with CRISPR immunity only targeting early lytic genes (∆RM, spcE) or late lytic genes (∆RM, spcL). Serial tenfold dilutions were made from the phage stock, and 2 µL of each dilution was spotted on the indicated bacterial lawn.
Extended Data Fig. 6 Kinetics of phage clearance by RM.
(a) Representative montage images from timelapse FROS experiments tracking ϕLS59 DNA during infection. LS1 cells with the indicated genotype harboring tetR-mCherry were infected with ϕLS59 tetOx60 and imaged every 5 minutes post-infection for 1 hour. Yellow carets indicate the presence of a phage DNA focus. Scale bar = 1 µm. (b) Quantitation of data in (a); percentage of cells that retained phage DNA foci at each time point after infection. Error bars represent standard error of the mean.
Extended Data Fig. 7 Phage A118 is sensitive to both RM and type VI CRISPR immunity.
(a) Region of the A118 genome targeted by natural and engineered type VI CRISPR spacers. (b) Plaque assay of A118 on on a set of L. seeligeri strains lacking RM and type VI CRISPR immunity (∆RM, nt spc), equipped with RM immunity only (WT, nt spc), or equipped with CRISPR immunity only targeting early lytic genes (∆RM, spcE). Serial tenfold dilutions were made from the phage stock, and 2 µL of each dilution was spotted on the indicated bacterial lawn. (c) Growth curves during A118 infection of LS1. Strains of the indicated genotype were infected at MOI = 1, OD600 = 0.01. OD600 was monitored for 20 hours after infection. Solid lines indicate wild-type, RM+ strains, dashed lines indicate ∆RM strains. Uninfected controls are shown in red. Error bars are standard error of the mean from 3 biological replicates.
Supplementary information
Supplementary Information
Supplementary Discussion and References.
Supplementary Table 1
Global RM Cas13 bioinformatics.
Supplementary Table 2
Strains, plasmids and oligonucleotides used in this study.
Supplementary Data 1
PacBio analysis report from RM1 deletion strain.
Supplementary Data 2
PacBio analysis report from RM2 deletion strain.
Source data
Source Data Fig. 3
Unprocessed northern blots.
Source Data Extended Data Fig. 4
Unprocessed agarose gels.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Williams, M.C., Reker, A.E., Margolis, S.R. et al. Restriction endonuclease cleavage of phage DNA enables resuscitation from Cas13-induced bacterial dormancy. Nat Microbiol 8, 400–409 (2023). https://doi.org/10.1038/s41564-022-01318-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41564-022-01318-2
This article is cited by
-
Phage Paride can kill dormant, antibiotic-tolerant cells of Pseudomonas aeruginosa by direct lytic replication
Nature Communications (2024)
-
The highly diverse antiphage defence systems of bacteria
Nature Reviews Microbiology (2023)