Abstract
Environmental enteropathy is a major contributor to growth faltering in millions of children in Africa and South Asia. We carried out a longitudinal, observational and interventional study in Lusaka, Zambia, of 297 children with stunting (aged 2–17 months at recruitment) and 46 control children who had good growth (aged 1–5 months at recruitment). Control children contributed data only at baseline. Children were provided with nutritional supplementation of daily cornmeal-soy blend, an egg and a micronutrient sprinkle, and were followed up to 24 months of age. Children whose growth did not improve over 4–6 months of nutritional supplementation were classified as having non-responsive stunting. We monitored microbial translocation from the gut lumen to the bloodstream in the cohort with non-responsive stunting (n = 108) by measuring circulating lipopolysaccharide (LPS), LPS-binding protein and soluble CD14 at baseline and when non-response was declared. We found that microbial translocation decreased with increasing age, such that LPS declined in 81 (75%) of 108 children with non-responsive stunting, despite sustained pathogen pressure and ongoing intestinal epithelial damage. We used confocal laser endomicroscopy and found that mucosal leakiness also declined with age. However, expression of brush border enzyme, nutrient transporter and mucosal barrier genes in intestinal biopsies did not change with age or correlate with biomarkers of microbial translocation. We propose that environmental enteropathy arises through adaptation to pathogen-mediated epithelial damage. Although environmental enteropathy reduces microbial translocation, it does so at the cost of impaired growth. The reduced epithelial surface area imposed by villus blunting may explain these findings.
Similar content being viewed by others
Main
Mortality in African children under five years of age has fallen over recent decades1,2 and much residual mortality is related to neonatal disorders or undernutrition3. Undernutrition, however, presents a frustrating paradox. It does not respond reliably to the provision of extra nutrients. This is most clearly seen in children with stunting, in whom nutritional supplementation consistently corrects only about 10% of the linear growth deficit4,5,6,7. Nutritional supplementation before or during pregnancy also delivers only a modest improvement in child linear growth8. Evidence now suggests that the refractory nature of linear growth failure is largely attributable to enteropathy present in undernutrition disorders9,10,11,12; enteropathy would also explain historical data regarding stunting13.
The term enteropathy refers to a global change in mucosal structure and function in the small intestine. While there is no consensus on the drivers of environmental enteropathy, some contributors to its pathogenesis are understood. At an ecological level, it is related to socio-economic conditions rather than latitude14, which is the principal reason for the renaming from ‘tropical’ to ‘environmental’. Onset begins after birth9,15. It is seasonal16, which strongly implicates environmental exposure, but the precise environmental noxae are uncertain. Environmental enteropathy resolved in Peace Corps volunteers after repatriation to the USA17 and resolved in immigrants to the UK in proportion to time since arrival18.
Enteropathogens (bacterial, protozoal and viral) have highly plausible roles as contributors to poor growth19,20. They cause epithelial damage, which is observed in children with growth failure21. Epithelial damage is a crucial step in enabling microbial translocation, which causes mucosal and systemic inflammation20.
In this study, we report that microbial translocation gradually diminishes in intensity despite ongoing stunting, which we interpret as adaptation to pathogen-mediated epithelial damage.
Results
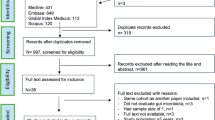
Description of the BEECH study
The Biomarkers of Environmental Enteropathy in Children (BEECH) study was a community-based longitudinal study of stunting in the Misisi, Chawama, Kuku and John Laing residential areas in Lusaka, which took place from August 2016 to June 2019. Children (n = 5,660) between 0 and 18 months of age were screened for low weight-for-age; 401 were identified as eligible because length-for-age (LAZ), weight-for-age (WAZ) or weight-for-length (WLZ) scores were <−2; 297 were recruited when their primary caregiver gave written consent. Children were then followed up every 2 weeks; 213 were still under follow-up at 24 months of age (Extended Data Fig. 1). All children were entered into a nutritional rehabilitation programme that provided a daily ration of high-energy protein supplement (corn-soy blend) porridge, micronutrient sprinkles22 (Nutromix; Hexagon Nutrition) and an egg23. The trajectories of LAZ and WLZ scores were evaluated after initiation of nutritional supplements. Those children (n = 191) whose LAZ scores were consistently <−2 over 4–6 months of observation were declared non-responders and underwent medical evaluation. In most children, no clinical explanation for non-responsive stunting (for example, cardiac disorder or tuberculosis) was found; endoscopy was offered in an attempt to find treatable causes for stunting. A total of 119 caregivers consented to endoscopy (Extended Data Fig. 1). At baseline, 69 children had wasting (WLZ, <−2) but by the date when non-response was declared, only 9 had wasting; thus, wasting responded better to nutritional intervention than stunting. Controls (n = 46) were simultaneously recruited by identifying children with good growth from the same community (Table 1).
Epithelial damage, microbial translocation and inflammation
Circulating concentrations of bacterial lipopolysaccharide (LPS), LPS-binding protein (LBP) and soluble CD14 (sCD14) were greater in children with stunting at baseline than in controls (Fig. 1; P = 0.0001 by Kruskal–Wallis test for all). Intestinal fatty acid-binding protein (iFABP) concentrations did not differ (Fig. 1). Since controls were younger than children with stunting at baseline, we confirmed that differences in LPS, LBP and sCD14 concentrations were statistically significant even if restricted to children under 9 months of age (P < 0.001, P = 0.007 and P < 0.001, respectively by Kruskal–Wallis test). However, circulating biomarker concentrations in children with non-responsive stunting thereafter showed two markedly divergent patterns: iFABP concentrations increased over the period from baseline to the age when non-response was declared; circulating LPS, LBP and sCD14 concentrations decreased in the same children with non-response (P < 0.001, P = 0.006 and P < 0.001, respectively by Wilcoxon signed-rank test; Fig. 1). Decreases in LPS, LBP and sCD14 were observed in 75, 64 and 86% of children, respectively, while iFABP increased in 63% (Extended Data Fig. 2). Since obviously age increased between baseline and non-response, we next analysed changes with age in biomarker concentrations. Biomarkers of microbial translocation (LPS, LBP, sCD14) were reduced when non-response was declared, irrespective of age, but iFABP concentrations were not reduced (Fig. 2). LAZ did not vary with age in children with non-responsive stunting but LAZ was inversely associated with iFABP concentrations (Extended Data Fig. 3). Biomarkers were not significantly different in the nine children with residual wasting on top of non-responsive stunting (data not shown).
Plasma concentrations of biomarkers in children with stunting at baseline (n = 297), when non-response was declared (n = 108) and in controls at baseline (n = 44). a–d, LPS (a); LBP (b), sCD14 (c) and iFABP (d). Differences between baseline samples in children with stunting and controls were significant for LPS, LBP and sCD14 (P = 0.0001 by two-sided Kruskal–Wallis test) but not iFABP. To compare those children who had both baseline samples and samples when non-response was declared, the Wilcoxon signed-rank test was used: LPS (P < 0.001); LBP (P = 0.006); sCD14 (P < 0.001); iFABP (P = 0.01). Several extreme values have been omitted from the graphs to allow easier visual assessment: 2 LPS values of over 3,000 EU ml−1 at baseline and 3 iFABP values of over 10 ng ml−1, two of which were at baseline. However, these values were included in all statistical analyses. The box plot shows the median, IQR and 5th and 95th centiles.
a–c, Plasma concentrations of LPS (a), LBP (b), sCD14 (c) and iFABP (d) in children sampled at different ages, some of whom were sampled twice. Values in children with stunting at baseline (n = 297) are shown in blue, when non-response was declared (n = 108) in magenta and in controls (n = 44) in cyan. Again, a small number of extreme values have been omitted to bring the y axis scale into an appropriate range.
Enteropathogen colonization
We detected enteropathogens considerably more frequently in children with stunting at baseline than in controls (Supplementary Table 1); children with stunting excreted up to 11 pathogens concurrently. Children with stunting under 9 months of age had a median of 4 (interquartile range (IQR) = 3–6) pathogens simultaneously; controls in this age band had only 1 (IQR = 0–3) pathogen (P = 0.0001). Pathogen detection did not diminish with age (Fig. 3a). Linear regression showed that age increased pathogen burden and confirmed that controls had two fewer pathogens per child (Supplementary Table 2). Logistic regression for individual pathogen positivity showed that controls had fewer pathogens when age was adjusted for; however, no difference was found for Cryptosporidium or Giardia (Supplementary Table 2). Other measures of potential microbiological contributors to stunting or epithelial damage (urinary aflatoxin M1 excretion24 and Helicobacter pylori seropositivity25) were also sustained or increased with age (Fig. 3b,c). Circulating iFABP concentrations were increased by 0.12 ng ml−1 for every additional enteric pathogen at baseline (β = 0.12; P = 0.02; Extended Data Fig. 4). In multivariable regression analysis, only Cryptosporidium infection was correlated with iFABP concentrations in the whole group (β = 1.35; P < 0.001), which included 37 children who were reported to have had diarrhoea in the 7 d before sampling. The association remained true in children who had not experienced any diarrhoea in stools within the previous 7 d (β = 1.37; P = 0.002).
Pathogen burden did not decrease in children with non-responsive stunting over the first 22 months of life. a, Proportion of stool samples positive for each pathogen shown in children with stunting in each of four quartiles of age, compared to controls (who were all aged 0–9 months). The proportion of positive stool sample totals was over 4.0 (400%) due to the high number of concurrent multiple pathogens detected in each sample. Some children submitted two samples at different time points. b, Continuing or increasing exposure to aflatoxin, measured as aflatoxin M1 (pg ml−1) in urine samples. c, Seropositivity to H. pylori increased with age, with 4% of children under 9 months of age being seropositive but 11% of children over that age positive. Absolute serological values also increased (β = 0.45; P = 0.005). The red line shows the cut-off for positivity (30 EU ml−1). Regressions were not adjusted for multiple testing.
Endoscopic biopsy and confocal laser endomicroscopy of mucosae
We performed oesophagogastroduodenoscopy in 118 children with non-responsive stunting and confocal laser endomicroscopy (CLE) in a subset of 75. None of these children had had diarrhoea in the 14 d before endoscopy. The time taken for initial fogging to clear from the confocal endomicroscope lens and the time while the probe tip was not in contact with the mucosa left a mean of 49 s (range 27–106) of assessable CLE video recording for each child. Fluorescein leakage was quantified both as the number of leakage events (plumes) and as the total proportion of imaged time during which luminal fluorescein was visible (Fig. 4); these measures were highly correlated (Extended Data Fig. 5). Interpreters of the videos were blinded to the clinical or research data from individual children. Fluorescein leakage varied from 0 to 100% of video time assessed (median = 29%, IQR = 16–46%). No sex difference was observed. Fluorescein leak, as a proportion of time imaged, declined with age (regression coefficient β = −0.022; P < 0.001) and was inversely correlated with crypt depth (ρ = −0.34; P = 0.01; Fig. 4).
a–d, Normal epithelium illuminated by fluorescein, with dark lumen (a), fluorescein-filled capillaries seen underlying the epithelium (b), plumes of fluorescein from an epithelial defect (c) and extensive leak of fluorescein from the epithelium (d). e, Reduction in epithelial barrier dysfunction (as measured by fluorescein leakage) with increasing age over the first 2 years of life in children with non-response (β = −0.022; P < 0.0001). f, Fluorescein leak was inversely correlated with crypt depth (β = −0.002; P = 0.03) but not villus height or epithelial surface area (data not shown). Regressions were not adjusted for multiple testing.
Three duodenal biopsies were taken from each child undergoing oesophagogastroduodenoscopy. Biopsies demonstrated severe enteropathy in these asymptomatic children (Fig. 5). Villus height (median = 180 µm, IQR = 144–216) and crypt depth (median = 171 µm, IQR = 147–203) were similar to measurements previously reported (median = 211 and 157 µm, respectively) in children with severe acute malnutrition in our centre21. Biopsies from two children showed total villus atrophy. Villus height, crypt depth and villus surface area, as measured in intestinal biopsies, did not change with age in children with non-responsive stunting.
a, Biopsy with tall villi, little inflammation and intact epithelium, but some early villus fusion apparent. b, Moderate enteropathy with villus blunting and epithelial damage (solid arrows) and a focus of chronic inflammation (marked by the asterisk). c, Severe enteropathy with subtotal villus blunting and areas of epithelial loss (solid arrows) and a focus of chronic inflammation (marked by the asterisk). d, Close-up of epithelial damage showing a microerosion characterized by the progressive loss of enterocyte height at the edges, which are shown by the hollow arrows. e, Morphometric analysis showing the measurements of villus height (black lines), crypt depth (yellow lines), villus surface area (white perimeter) and length of muscularis mucosae (green line), which is the denominator for calculating the epithelial surface area. a–d, Scale bars, 100 μm.
Transcriptomic analysis of microbial translocation
Since intestinal-type alkaline phosphatase (ALPI), expressed in the intestinal microvillus brush border, is known to hydrolyze LPS26, we sought evidence that specific expression of the ALPI gene could explain changes in circulating LPS (Supplementary Table 3) using RNA sequencing on biopsies from 30 children. No significant relationship was found between expression and age or between ALPI expression and LPS. However, circulating LPS concentrations were significantly inversely correlated with three other brush border enzymes (Supplementary Table 3): maltase (ρ = −0.45; P = 0.03); folate hydrolase (ρ = −0.37; P < 0.05); and angiotensin converting enzyme (ρ = −0.45; P = 0.03). In general, expression of these enzymes was highly correlated (Extended Data Fig. 6).
To determine if changes in the expression of genes encoding specific barrier functions could explain changes in the microbial translocation observed, we selected 73 transcripts of barrier-relevant genes (that is, those affecting mucus secretion or stability, tight junction function or antimicrobial activity (Supplementary Table 4). None of these transcripts varied significantly with age; therefore, they cannot explain the observed decline in microbial translocation. Circulating LPS concentrations were inversely correlated with the expression of MUC13 and MUC17 (ρ = −0.38 and −0.37, respectively; P = 0.04 for both) and LBP concentrations were inversely associated with MUC4 (ρ = −0.45; P = 0.01). Circulating sCD14 also inversely varied with claudin-4 (ρ = −0.46; P = 0.01) expression. Taken together, these correlations emphasize the importance of barrier proteins in controlling microbial translocation but do not explain the inferred decrease in translocation with increasing age.
To determine if absorptive function reflects the adaptive response, we analysed gene expression in a set of 21 key solute carrier transcripts, selected to represent the uptake of key nutrients and on the basis of abundance (Supplementary Table 5). None correlated with age but several inversely correlated with translocation markers and iFABP, as would be expected in damaged epithelium.
HIV and study population
At recruitment, 10 and 83 children were HIV-infected or exposed but uninfected, respectively (Supplementary Table 6). LAZ was lower in children who were HIV-exposed but uninfected and lower still in children with active HIV infection (P = 0.006 by non-parametric trend test; Supplementary Table 6). Circulating markers of translocation did not vary significantly with HIV status (Supplementary Table 6). The effect of HIV on confocal endomicroscopy scores, villus height and crypt depth could not be evaluated because, by chance, only one HIV seropositive child underwent endoscopy.
Discussion
Understanding the development of environmental enteropathy in children living in unsanitary, impoverished environments is of prime importance if we are to devise methods to prevent it. Our findings in Lusaka show that young children with non-responsive stunting have continuous, intense exposure to enteropathogens and remarkably severe ongoing enteropathy, with greatly reduced villus height and villus:crypt ratios barely greater than unity. Three biomarkers of microbial translocation all showed markedly higher levels at baseline in children with stunting but, counter-intuitively, reverted towards control values over the next year or more despite continuing, often severe, growth faltering. Children who did not respond to nutritional supplementation had significantly lower microbial translocation marker levels than 6–12 months previously. Consistent with this, leakage of fluorescein, imaged using CLE, also declined with increasing age. We propose that the pathology we report indicates that environmental enteropathy is an adaptive response that reduces the potentially lethal exposure to microbial translocation that occurs owing to polymicrobial pathogen-induced epithelial damage. Such an adaptation might confer a survival advantage in settings where pathogen-mediated intestinal damage is a substantial burden.
Environmental enteropathy is associated with high, sustained circulating plasma iFABP concentrations indicative of continuing structural damage to the epithelium. Indeed, iFABP concentrations increased over time in children who failed to respond to nutritional interventions and circulating iFABP was inversely associated with LAZ. iFABP was positively associated with pathogen burden, especially cryptosporidiosis. Taken together with histological and other evidence, this clearly indicates ongoing epithelial damage associated with enteropathogens and with stunting.
We considered several explanations for reduced epithelial leakage concurrent with unexpectedly reduced biomarkers of microbial translocation in children with non-responsive stunting. Epithelial healing is one candidate but the persistence of elevated iFABP concentrations argues against this. Improved tight junction integrity might also explain the observed change but we observed no change in the specific expression of tight junction protein genes with age. Maturation of the microbiota might underlie the apparent adaptation because immaturity of the gut microbiota is associated with undernutrition27,28 and restoration of microbial communities increases colonization resistance to pathogens29. Recent evidence from the adoptive transfer of duodenal microbiota from children with stunting in Bangladesh into gnotobiotic mice identifies its role in the health of the mucosa30. Taken together with our data, this seems to suggest that the health of the mucosa depends on the composition, and presumably metabolic activity, of the microbial communities in the small intestinal lumen and mucus layer. We speculate that quorum sensing may mediate the interplay between commensals and pathobionts, which is now an area ripe for further study. One of the great unknowns in microbial translocation is the fractional extraction of pathogen-associated molecular patterns by Kupffer cells in the hepatic sinusoids31. An increase in this fractional extraction would explain reduced translocation but this would not explain the reduction in leakage observed by CLE and it is most likely that the adaptive response is located in the intestinal mucosa.
Our data raise the intriguing possibility that reduced absorptive surface area is the reason for diminishing translocation. Such an organ response would reduce overall translocation and fluorescein leakage. The lack of detectable change in villus morphology with age in the biopsy sample is compatible with this process since the small pinch biopsies that can be obtained in young children are unlikely to represent fully changes that occur across the very large surface of the whole small intestine. Biopsies, which often have only 6–20 measurable villi, are not ideal to measure surface area due to this sampling problem. Furthermore, transcriptomic analysis uses a measure of cell mass as its denominator and changes in total surface area may lead to changes in the absolute number of solute transporter or mucin or defensin molecules without a measurable change in the specific expression (fragments per kilobase of transcript per million mapped reads) value. Changes in surface area could also be attributable to changes in the surface area of the microvilli32, which are difficult to measure. Such an adaptation, reduced total surface area caused by blunting of villi and microvilli, would necessarily incur a cost, that is, reduced nutrient absorptive capacity. In other words, adaptation to enteropathy in children with refractory stunting in Lusaka represents an extensive response of the small bowel, which is not apparent in the intensive assessment of pinch biopsies necessarily limited to very small axial lengths.
Loss of surface area would explain why children with stunting do not respond well to nutrient supplementation and we propose that this is the most parsimonious explanation for our findings.
We recognize that our interpretation of these data is constrained by several important limitations. Most importantly, because we have no ethical justification for endoscopy on children who are healthy, we have almost no information on healthy children of the same ages from the same region. Many mothers of healthy children were also unwilling to participate once told their children were growing well, which explains why a small fraction of the potential controls actually consented. It is also a limitation that morphometry can only be performed on a few very small biopsies from the second or third part of the duodenum; it is difficult to believe that these can fully represent the overall state of the mucosa of the distal duodenum, jejunum and ileum. The carriage rate of enteropathogens, measured by PCR using the xTAG Gastrointestinal Pathogen Panel (GPP), was higher than studies from other settings. However, it must be emphasized that our study was entirely conducted in a very disadvantaged community with poor sanitation and very few resources. We are aware of only one other study in Zambia using the same diagnostic tool33; that study included rural areas and a broader range of urban socio-economic conditions and found carriage rates about half of those reported in this study. Molecular diagnosis has been widely used for analysis of enteropathogen burden and the xTAG GPP panel has been reported to deliver accurate pathogen detection34,35. The eight-country MAL-ED study demonstrated the usefulness of PCR-based diagnosis36 but as always the biological significance of low-intensity infections is unclear.
The four biomarkers on which we have focused all report different facets of pathophysiology. iFABP is an intracellular, mainly cytosolic protein of the enterocyte involved in long-chain fatty acid absorption37, which is released into the bloodstream by epithelial damage and is used as a biomarker of coeliac disease and environmental enteropathy38. LPS, a cell wall component of Gram-negative bacteria, is measured directly39 using the Limulus amoebocyte lysate assay or indirectly using host-response molecules such as LBP and sCD14. These host molecules belong to the Toll-like receptor 4 complex on monocytes and macrophages, one of several LPS recognition systems40. If it is confirmed that the small intestinal mucosa adapts over time to a hostile environment through reduced microbial translocation, this might explain why some studies show a weak relationship between biomarkers of enteropathy based on microbial translocation and growth failure41,42.
CLE allows direct imaging of the small intestinal mucosa and fluorescein efflux allows quantification of epithelial leakage in vivo43,44. The reduction in fluorescein leakage with age is consistent with the reduction in microbial translocation shown using three blood biomarkers. Fluorescein has similar molecular size to lactulose, a widely used marker of paracellular permeation45, and is emerging as an alternative way of assessing permeability46. We observed multiple fluorescein plumes emitted from the duodenal epithelium, which we interpret as evidence of breaches in epithelial continuity (Figs. 4 and 5). Fluorescein leakage measured endoscopically would reflect loss of epithelial continuity from microerosions but would also be reduced by any reduction in the surface area of damaged enterocytes. We previously reported fluorescein leakage in adults with environmental enteropathy from the same community in Lusaka as the children we report in this study43,44. It is of interest that increased crypt depth was associated with reduced fluorescein leakage. This might reflect the proliferative response, since crypt hypertrophy is generally a marker of increased proliferation47, and therefore greater capacity to replace damaged cells on the villus epithelium.
We propose that environmental enteropathy is an adaptation that sacrifices long-term nutritional health and stature in favour of short-term survival. If so, epithelial healing might emerge as the most essential requirement for catch-up growth. Therapies that chelate pathogen-associated molecular patterns might prove beneficial since they would rapidly reduce mucosal and systemic inflammation.
Methods
BEECH study approval and processes
The study was approved by the University of Zambia Biomedical and Research Committee (ref. 006-02-16, 31 May 2016). Informed written consent was obtained from the parents or primary caregivers of the children and the study was conducted in compliance with the Declaration of Helsinki (version 2008). The community where this study was entirely conducted is Misisi, a densely populated residential area just south of central Lusaka. Houses are constructed of concrete blocks with iron sheet roofing, sanitation is poor (1 pit latrine per 60 residents) and water supply is from communal standpipes. Domestic animals are free to roam around but few householders keep pets so the numbers of chickens, cats and dogs are low, but rats are frequent pests. In general, consumption of animal source foods is infrequent, but a daily egg was provided by the study team for each participating child.
Children were screened in the community using only weight measurements because length measurements in the community are impractical. Children were monitored every two weeks from recruitment to discharge but length/height was measured only monthly. Non-response was defined as failure to achieve a positive gradient in LAZ over at least 4 months, together with LAZ consistently below −2. In two cases, children booked for endoscopy showed LAZ greater than −2 on the last assessment but endoscopy carried out as part of the overall profile of LAZ was consistently non-responsive until that isolated measurement. Controls (n = 46) were simultaneously recruited by identifying children with good growth (LAZ, WAZ and WLZ all greater than −2 at recruitment, but preferably with z-scores all greater than −1) from the same community (Table 1). These children were provided with supplementary food for compassionate reasons but did not undergo endoscopy. In September 2016, recruitment began with inclusion restricted to children under 6 months of age; after January 2017, inclusion criteria were broadened to include undernourished children up to 18 months of age. Recruitment of controls was unchanged at up to six months of age. Recruitment of controls was only initiated after that change had been made. Thus, the final dataset included undernourished children recruited at 0–18 months of age and controls recruited at 0–6 months of age. The period of observation encompassed all seasons of the year since environmental enteropathy is a seasonal disorder16. Pathogen analysis was conducted within three months of recruitment. Biomarkers were measured within three months of recruitment and again when non-response was declared.
Growth and anthropometry
Children were fully assessed for growth faltering every month by trained study nurses who performed anthropometry in triplicate. Measurements were immediately entered into the WHO Anthro v3.2.2 software for calculation of precise WLZ, LAZ and WAZ scores and checked for plausibility and consistency by a study paediatrician (BA or KC). The instruments used included infant and mobile scales (seca 384 and 874) for weight, infantometer (seca 416) and UNICEF stadiometer for length.
Pathogen analysis
Stool samples were collected from cases at baseline and after 3 months of nutritional supplementation, and at baseline only for the controls, and stored (−80 °C) before enteropathogen analysis using the xTAG GPP kit (Luminex Corporation), which qualitatively detects viral, parasitic and bacterial nucleic acids in stool samples. A total of 286 Luminex stool results from cases and controls were available for this analysis; 198 were baseline samples and 88 were repeat samples collected approximately 3 months later.
Biomarkers of enteropathy and microbial translocation
Blood samples were taken from children with stunting and from controls at recruitment and in children with stunting with non-response to the nutritional intervention at the time of endoscopy. iFABP is a protein constitutively expressed in intestinal epithelial cells. It is released by cell damage and circulating concentrations reflect the severity of epithelial damage48,49. It was measured in plasma by enzyme-linked immunosorbent assay (ELISA; Cambridge Bioscience). LPS, LBP and sCD14 were used as markers of microbial translocation resulting from impairment of the gut barrier function. For LPS analysis, the pyrochrome Limulus amoebocyte lysate assay (Associates of Cape Cod) kit was used. Human LBP and sCD14 were assayed by ELISA (R&D Systems). Helicobacter serology was evaluated in serum using total antibodies to H. pylori by ELISA (BIOHIT HealthCare) and aflatoxin M1 was measured in urine, also by ELISA (Helica Biosystems).
Endoscopy and CLE
Once children were declared to have non-response, they were booked to have endoscopy with a Pentax EG-2490k paediatric gastroscope under ketamine-based sedation administered by an anaesthetist. Before endoscopy, several checks were carried out to identify conditions that might predispose to adverse events, including full blood count and measurement of prothrombin time (as international normalized ratio). During endoscopy, an injection of 2 ml 1% fluorescein in normal saline was administered, followed by a 60-s video recording using a Cellvizio confocal endomicroscopy probe (Mauna Kea Technologies). After clearing of initial lens fogging, video was recorded continuously. Endoscopic biopsies were obtained from the second part of the duodenum using standard disposable biopsy forceps and three biopsies were placed in normal saline to facilitate orientation under a dissecting microscope, followed by immersion in formal saline on cellulose acetate strips (0.45-μm pore; catalogue no. 11106-30-N, Sartorius AG). The maximum period before fixation was 10 min, although usually less. Biopsies were embedded in paraffin wax and stained with haematoxylin and eosin, then imaged on an Olympus VS120 scanning microscope. Morphometry was performed as described previously44 (Fig. 5).
RNA sequencing
RNA sequencing (RNA-seq) was performed on biopsies from the first 30 consecutive children to be declared to have non-responsive stunting, without selection. Two small intestinal biopsies were immediately snap-frozen in liquid nitrogen before storage at −80 °C. RNA was extracted using TRIzol (Invitrogen) followed by silica column purification (RNeasy Mini Kit; QIAGEN) and quantified using a NanoDrop spectrophotometer (ND-2000C; Thermo Fisher Scientific) before transport to the Beijing Genomics Institute. RNA quality control was performed using an Agilent 2100 Bioanalyzer and ABI StepOnePlus Real-Time PCR System. For RNA-seq preparation, total RNA was treated with DNase I followed by messenger RNA enrichment using oligo-deoxythymine-labelled beads and ligation of sequencing adaptors to the enriched mRNA fragments. RNA-seq was carried out using an Illumina HiSeq 2000 Sequencing System with 50 base pair reads. Filtering steps included removing reads with adaptors, removing reads where unknown bases were >10% and removing low-quality reads (percentage of low-quality bases >50%). The proportion of clean reads was never <99% and usually >99.6%. After low-quality reads were removed and adaptor sequences trimmed, 3.3 billion clean reads were obtained, with an average of 165 million reads per sample. mRNA tissue content was expressed as fragments per kilobase mapped values and therefore indicates gene expression per unit mass of tissue sequenced.
Data analysis
Biomarkers were assessed at recruitment (children with stunting and controls) and then once non-response was declared (children with stunting only). Biomarkers were generally non-normally distributed so are presented as the median and IQR. Statistical testing was performed using the Kruskal–Wallis test for children with stunting–controls comparisons, Wilcoxon signed-rank test for repeated tests (baseline compared to non-response in children with stunting) and Fisher’s exact test for categorical variables; all tests were two-sided. Correlations used Spearman’s non-parametric correlation coefficient (ρ) and linear regression was used to assess the dependence of selected variables on age (the regression coefficient β is quoted where significant at P < 0.05). No correction for multiple hypothesis testing was carried out for gene correlations; it was clear that few of these correlations (Supplementary Tables 3–5) were significant and these are listed mainly to demonstrate that adaptation does not appear to operate at the level of specific gene expression. In some graphs, extreme values have been omitted merely to bring data into a more visually acceptable scale; no data were omitted in any analysis.
Pathogens were compared in children with stunting and controls and analysed by quartile of age in children with stunting, as well as total the number of pathogens present in the stool sample from each child. Linear regression of total pathogen numbers and logistic regression of individual pathogen carriage were analysed in a subset of samples restricted to the first sample collected to confirm the effect of age and control status on pathogen carriage (Supplementary Table 2). The relationship between infecting pathogen and circulating iFABP was examined in a backwards stepwise linear regression model with all pathogens with a frequency greater than n = 5 included as the independent variables and iFABP (raw and square root-transformed) as the dependent variable; the statistical associations were identical, so the regressions of raw iFABP are reported. Analysis of endomicroscopy videos was performed in two ways. First, by assessing each still frame (10 per second) for the presence of fluorescein leakage (Fig. 1). We have previously reported that fluorescein leakage is the most reliable (lowest inter-observer variation) measure of epithelial damage44. Images with movement artefact and images where the epithelium could not be clearly visualized were not evaluated. Images with fluorescein leakage were expressed as a proportion of all images evaluated. Second, continuous play of each video recording was viewed to measure the proportion of time recorded during which fluorescein was seen to be leaking through the epithelium. These two measurement methods, evaluated by different observers, were moderately correlated (ρ = 0.44; P = 0.0001; Extended Data Fig. 5). Both measures were used in all analyses and similar results were obtained; however, only the second is reported in the Results for economy of space.
Reporting Summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
Data have been deposited with Dryad (doi:10.5061/dryad.zkh18937z; https://datadryad.org/stash/share/fYdcG3Ao8fnLwUbwF9N4RIJ4BYfvToSsg-iYuydWK34). This project contains the following data: data file 1—CSV file of pathogens in stool samples from children with stunting and their controls; data file 2—CSV file of biomarkers from children with stunting and their controls. Data are available under the terms of the Creative Commons Zero ‘No Rights Reserved’ data waiver (CC0 1.0 Public Domain Dedication). Transcriptomic data have been deposited with the Gene Expression Omnibus under accession no. 162630.
Change history
08 December 2021
A Correction to this paper has been published: https://doi.org/10.1038/s41564-021-01042-3
References
Mejía-Guevara, I., Zuo, W., Bendavid, E., Li, N. & Tuljapurkar, S. Age distribution, trends, and forecasts of under-5 mortality in 31 sub-Saharan African countries: a modeling study. PLoS Med. 16, e1002757 (2019).
Masanja, H. et al. Factors associated with the decline in under five diarrhea mortality in Tanzania from 1980–2015. J. Glob. Health 9, 020806 (2019).
Black, R. E. et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet 382, 427–451 (2013).
Dewey, K. G. & Adu-Afarwuah, S. Systematic review of the efficacy and effectiveness of complementary feeding interventions in developing countries. Matern. Child Nutr. 4, 24–85 (2008).
Luby, S. P. et al. Effects of water quality, sanitation, handwashing, and nutritional interventions on diarrhoea and child growth in rural Bangladesh: a cluster randomised controlled trial. Lancet Glob. Health 6, e302–e315 (2018).
Null, C. et al. Effects of water quality, sanitation, handwashing, and nutritional interventions on diarrhoea and child growth in rural Kenya: a cluster-randomised controlled trial. Lancet Glob. Health 6, e316–e329 (2018).
Humphrey, J. H. et al. Independent and combined effects of improved water, sanitation, and hygiene, and improved complementary feeding, on child stunting and anaemia in rural Zimbabwe: a cluster-randomised trial. Lancet Glob. Health 7, e132–e147 (2019).
Hambidge, K. M. et al. A multicountry randomized controlled trial of comprehensive maternal nutrition supplementation initiated before conception: the Women First trial. Am. J. Clin. Nutr. 109, 457–469 (2019).
Keusch, G. T. et al. Implications of acquired environmental enteric dysfunction for growth and stunting in infants and children living in low- and middle-income countries. Food Nutr. Bull. 34, 357–364 (2013).
Prendergast, A. & Kelly, P. Enteropathies in the developing world; neglected effects on global health. Am. J. Trop. Med. Hyg. 86, 756–763 (2012).
Guerrant, R. L., DeBoer, M. D., Moore, S. R., Scharf, R. J. & Lima, A. A. M. The impoverished gut: a triple burden of diarrhoea, stunting and chronic disease. Nat. Rev. Gastroenterol. Hepatol. 10, 220–229 (2013).
Owino, V. et al. Environmental enteric dysfunction and growth failure/stunting in global child health. Pediatrics 138, e20160641 (2016).
Hermanussen, M., Bogin, B. & Scheffler, C. Stunting, starvation and refeeding: a review of forgotten 19th and early 20th century literature. Acta Paediatr. 107, 1166–1176 (2018).
Menzies, I. S. et al. Geography of intestinal permeability and absorption. Gut 44, 483–489 (1999).
Chacko, C. J., Paulson, K. A., Mathan, V. I. & Baker, S. J. The villus architecture of the small intestine in the tropics: a necropsy study. J. Pathol. 98, 146–151 (1969).
Kelly, P. et al. Responses of small intestinal architecture and function over time to environmental factors in a tropical population. Am. J. Trop. Med. Hyg. 70, 412–419 (2004).
Lindenbaum, J. Tropical enteropathy. Gastroenterology 64, 637–652 (1973).
Wood, G. M., Gearty, J. C. & Cooper, B. T. Small bowel morphology in British Indian and Afro-Caribbean subjects: evidence of tropical enteropathy. Gut 32, 256–259 (1991).
Salazar-Lindo, E. et al. Intestinal infections and environmental enteropathy: Working Group report of the second World Congress of Pediatric Gastroenterology, Hepatology, and Nutrition. J. Pediatr. Gastroenterol. Nutr. 39, S662–S669 (2004).
Kosek, M. N. et al. Causal pathways from enteropathogens to environmental enteropathy: findings from the MAL-ED birth cohort study. EBioMedicine 18, 109–117 (2017).
Amadi, B. et al. Impaired barrier function and autoantibody generation in malnutrition enteropathy in Zambia. EBioMedicine 22, 191–199 (2017).
Suchdev, P. S., Jefferds, M. E. D., Ota, E., da Silva Lopes, K. & De-Regil, L. M. Home fortification of foods with multiple micronutrient powders for health and nutrition in children under two years of age. Cochrane Database Syst. Rev. 2, CD008959 (2020).
Iannotti, L. L. et al. Eggs in early complementary feeding and child growth: a randomized controlled trial. Pediatrics 140, e20163459 (2017).
Smith, L. E. et al. The potential role of mycotoxins as a contributor to stunting in the SHINE trial. Clin. Infect. Dis. 61, S733–S737 (2015).
Dror, G. & Muhsen, K. Helicobacter pylori infection and children’s growth: an overview. J. Pediatr. Gastroenterol. Nutr. 62, e48–e59 (2016).
Bilski, J. et al. The role of intestinal alkaline phosphatase in inflammatory disorders of gastrointestinal tract. Mediators Inflamm. 2017, 9074601 (2017).
Subramanian, S. et al. Persistent gut microbiota immaturity in malnourished Bangladeshi children. Nature 510, 417–421 (2014).
Blanton, L. V., Barratt, M. J., Charbonneau, M. R., Ahmed, T. & Gordon, J. I. Childhood undernutrition, the gut microbiota, and microbiota-directed therapeutics. Science 352, 1533 (2016).
Stecher, B., Berry, D. & Loy, A. Colonization resistance and microbial ecophysiology: using gnotobiotic mouse models and single-cell technology to explore the intestinal jungle. FEMS Microbiol. Rev. 37, 793–829 (2013).
Chen, R. Y. et al. Duodenal microbiota in stunted undernourished children with enteropathy. N. Engl. J. Med. 383, 321–333 (2020).
Dixon, L. J., Barnes, M., Tang, H., Pritchard, M. T. & Nagy, L. E. Kupffer cells in the liver. Compr. Physiol. 3, 785–797 (2013).
Phillips, A. D. et al. Microvillous surface area in secondary disaccharidase deficiency. Gut 21, 44–48 (1980).
Chisenga, C. C. et al. Aetiology of diarrhoea in children under five in Zambia detected using Luminex xTAG gastrointestinal pathogen panel. Pediatr. Infect. Dis. 3, 8 (2018).
Perry, M. D., Corden, S. A. & Howe, R. A. Evaluation of the Luminex xTAG Gastrointestinal Pathogen Panel and the Savyon Diagnostics Gastrointestinal Infection Panel for the detection of enteric pathogens in clinical samples. J. Med. Microbiol. 63, 1419–1426 (2014).
Kellner, T. et al. Comparative evaluation of enteric bacterial culture and a molecular multiplex syndromic panel in children with acute gastroenteritis. J. Clin. Microbiol. 57, e00205-19 (2019).
Rogawski, E. T. et al. Use of quantitative molecular diagnostic methods to investigate the effect of enteropathogen infections on linear growth in children in low-resource settings: longitudinal analysis of results from the MAL-ED cohort study. Lancet Glob. Health 6, e1319–e1328 (2018).
Cifarelli, V. & Abumrad, N. A. Intestinal CD36 and other key proteins of lipid utilization: role in absorption and gut homeostasis. Compr. Physiol. 8, 493–507 (2018).
Zambruni, M. et al. Stunting is preceded by intestinal mucosal damage and microbiome changes and is associated with systemic inflammation in a cohort of Peruvian infants. Am. J. Trop. Med. Hyg. 101, 1009–1017 (2019).
Kaonga, P. et al. Direct biomarkers of microbial translocation correlate with immune activation in adult Zambians with environmental enteropathy and hepatosplenic schistosomiasis. Am. J. Trop. Med. Hyg. 97, 1603–1610 (2017).
Mazgaeen, L. & Gurung, P. Recent advances in lipopolysaccharide recognition systems. Int. J. Mol. Sci. 21, 379 (2020).
Benzoni, N. et al. Plasma endotoxin core antibody concentration and linear growth are unrelated in rural Malawian children aged 2–5 years. BMC Res. Notes 8, 258 (2015).
Harper, K. M., Mutasa, M., Prendergast, A. J., Humphrey, J. & Manges, A. R. Environmental enteric dysfunction pathways and child stunting: a systematic review. PLoS Negl. Trop. Dis. 12, e0006205 (2018).
Kelly, P. et al. Endomicroscopic and transcriptomic analysis of impaired barrier function and malabsorption in environmental enteropathy. PLoS Negl. Trop. Dis. 10, e0004600 (2016).
Louis-Auguste, J. et al. Tryptophan, glutamine, leucine, and micronutrient supplementation improves environmental enteropathy in Zambian adults: a randomized controlled trial. Am. J. Clin. Nutr. 110, 1240–1252 (2019).
Ordiz, M. I. et al. Interpretation of the lactulose:mannitol test in rural Malawian children at risk for perturbations in intestinal permeability. Exp. Biol. Med. (Maywood) 243, 677–683 (2018).
Dorshow, R. B. et al. Measurement of gut permeability using fluorescent tracer agent technology. Sci. Rep. 7, 10888 (2017).
Ferreira, R. C. et al. Changes in the rate of crypt epithelial cell proliferation and mucosal morphology induced by a T-cell-mediated response in human small intestine. Gastroenterology 98, 1255–1263 (1990).
Vreugdenhil, A. C. et al. Additional value of serum I-FABP levels for evaluating celiac disease activity in children. Scand. J. Gastroenterol. 46, 1435–1441 (2011).
Adriaanse, M. P. M. et al. Progress towards non-invasive diagnosis and follow-up of celiac disease in children; a prospective multicentre study to the usefulness of plasma I-FABP. Sci. Rep. 7, 8671 (2017).
Acknowledgements
We thank our endoscopy nurses and anaesthetists for assistance with the endoscopy procedures and to R. Chilengi and staff at the Centre for Infectious Disease Research in Zambia laboratory for work on the xTAG pathogen analysis. We thank R. Feldman, I. Sanderson, P. Tarr, D. Denno, P. Sullivan and K. Jones for helpful discussions. We thank T. Wylie, N. Shaikh and M. Ndao for invaluable technical support. This work was supported by the Bill & Melinda Gates Foundation, through the Environmental Enteric Dysfunction Consortium (grant no. OPP 1066118).
Author information
Authors and Affiliations
Contributions
B.A. and P.K. designed the study. B.A., K. Chandwe, R.B. and K. Chifunda designed the data collection processes, clinical follow-up procedures and data collection. K.Z., E.B., C.M., S.M. and S.S. performed the biomarker analyses. L.K., S.C., M.C., K.Z., K. Chandwe and K.V. were responsible for data management. P.K. and K.V. analysed the data and P.K. wrote the first draft of the manuscript. All authors contributed to checking and revising later versions of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Microbiology thanks the anonymous reviewers for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Flowchart of screening, recruitment and follow-up.
In September 2016, recruitment began with inclusion restricted to children under 6 months of age, but after January 2017 inclusion criteria were broadened to include undernourished children up to 18 months of age; recruitment of controls remained unchanged at up to 6 months of age. Recruitment of controls was only initiated after that change had been made. Thus the final dataset included undernourished children recruited at 0–18 months of age and controls recruited at 0–6 months of age. From March 2017, recruitment of cases and controls proceeded simultaneously, encompassing 2 full annual seasonal cycles. Pathogen analysis was conducted within 3 months of recruitment. Biomarkers were measured within 3 months of recruitment and again when non-response was declared.
Extended Data Fig. 2 Biomarkers at baseline and non-response.
Scatter plots of baseline values of biomarkers against values at non-response in the same child. The black line shown is the line of identity, so points above and to the left (coloured in navy) represent children whose baseline values were higher than when non-response was declared. Conversely, points below and to the right of the line denote children whose values had increased by the date of non-response. For the biomarkers of microbial translocation (LPS, LBP and sCD14) the proportions which showed a decrease were 75%, 64% and 86% respectively, whereas iFABP increased in 63%. LPS, lipopolysaccharide; LBP, LPS binding protein; sCD14, soluble CD14; iFABP, intestinal fatty acid binding protein.
Extended Data Fig. 3 Length for age z score in relation to age and epithelial damage.
a, Absence of regression of LAZ on age; the regression line is shown but was not significant (P = 0.36). b, Intestinal fatty acid binding protein (iFABP) was inversely correlated with LAZ score at the time when endoscopy was performed (ρ = −0.15; P = 0.003). Using linear regression to estimate the dependence of LAZ on iFABP, despite the non-normal distribution of iFABP, each unit ng/ml increase in FABP was associated with 0.08 z score reduction in LAZ (β = −0.08; P = 0.01). None of the biomarkers of microbial translocation correlated with LAZ.
Extended Data Fig. 4 Relationship between iFABP and number of pathogens detected concurrently.
Intestinal Fatty Acid Binding Protein (iFABP) was related with the total number of pathogens carried at points in time when measurements were made (regression coefficient β = 0.12; P = 0.02).
Extended Data Fig. 5 Confocal laser endomicroscopy imaging validation.
Concordance between confocal laser endomicroscopy leakage of fluorescein from systemic circulation into gut lumen, assessed using either incidence leakage events or proportion of time imaged.
Extended Data Fig. 6 Brush border enzyme gene expression.
Brush border enzymes were strongly correlated: examples shown are (a) sucrase isomaltase and dipeptidylpeptidase IV (DPP4; ρ = 0.78; P < 0.0001); b, maltase glucoamylase (MGAM) and alkaline phosphatase (ALPI; ρ = 0.65; P = 0.0001).
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Amadi, B., Zyambo, K., Chandwe, K. et al. Adaptation of the small intestine to microbial enteropathogens in Zambian children with stunting. Nat Microbiol 6, 445–454 (2021). https://doi.org/10.1038/s41564-020-00849-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41564-020-00849-w
This article is cited by
-
Intestine bacterial community affects the growth of the Pacific white shrimp (Litopenaeus vannamei)
Applied Microbiology and Biotechnology (2024)
-
Factors associated with stunting: gut inflammation and child and maternal-related contributors among under-five children in Hawassa City, Sidama Region, Ethiopia
BMC Nutrition (2023)
-
Risk factors for inpatient mortality among children with severe acute malnutrition in Zimbabwe and Zambia
European Journal of Clinical Nutrition (2023)
-
The intersection of undernutrition, microbiome, and child development in the first years of life
Nature Communications (2023)
-
The gut microbiome and early-life growth in a population with high prevalence of stunting
Nature Communications (2023)