Abstract
Transmission of Plasmodium falciparum malaria parasites occurs when nocturnal Anopheles mosquito vectors feed on human blood. In Africa, where malaria burden is highest, bednets treated with pyrethroid insecticide were highly effective in preventing mosquito bites and reducing transmission, and essential to achieving unprecedented reductions in malaria until 2015 (ref. 1). Since then, progress has stalled2, and with insecticidal bednets losing efficacy against pyrethroid-resistant Anopheles vectors3,4, methods that restore performance are urgently needed to eliminate any risk of malaria returning to the levels seen before their widespread use throughout sub-Saharan Africa5. Here, we show that the primary malaria vector Anopheles gambiae is targeted and killed by small insecticidal net barriers positioned above a standard bednet in a spatial region of high mosquito activity but zero contact with sleepers, opening the way for deploying many more insecticides on bednets than is currently possible. Tested against wild pyrethroid-resistant A. gambiae in Burkina Faso, pyrethroid bednets with organophosphate barriers achieved significantly higher killing rates than bednets alone. Treated barriers on untreated bednets were equally effective, without significant loss of personal protection. Mathematical modelling of transmission dynamics predicted reductions in clinical malaria incidence with barrier bednets that matched those of ‘next-generation’ nets recommended by the World Health Organization against resistant vectors. Mathematical models of mosquito–barrier interactions identified alternative barrier designs to increase performance. Barrier bednets that overcome insecticide resistance are feasible using existing insecticides and production technology, and early implementation of affordable vector control tools is a realistic prospect.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The hut trial dataset generated during the current study is available at Dryad Digital Repository (https://doi.org/10.5061/dryad.hqbzkh1b7). All data analysed during this study are available as described in the paper. All other data supporting the findings of this study are available within the article and its Supplementary Information files or are available from the authors on reasonable request.
Code availability
Data handling scripts and video segmentation and tracking software are available from the authors on reasonable request.
References
Bhatt, S. et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature 526, 207–211 (2015).
World Malaria Report 2018 (WHO, 2018).
Protopopoff, N. et al. Effectiveness of a long-lasting piperonyl butoxide-treated insecticidal net and indoor residual spray interventions, separately and together, against malaria transmitted by pyrethroid-resistant mosquitoes: a cluster, randomised controlled, two-by-two factorial design trial. Lancet 391, 1577–1588 (2018).
Churcher, T. S., Lissenden, N., Griffin, J. T., Worrall, E. & Ranson, H. The impact of pyrethroid resistance on the efficacy and effectiveness of bednets for malaria control in Africa. eLife 5, e16090 (2016).
Hemingway, J. The way forward for vector control. Science 358, 998–999 (2017).
Pryce, J., Richardson, M. & Lengeler, C. Insecticide-treated nets for preventing malaria. Cochrane Database Syst. Rev. 11, CD00063 (2019).
Ranson, H. & Lissenden, N. Insecticide resistance in African Anopheles mosquitoes: a worsening situation that needs urgent action to maintain malaria control. Trends Parasitol. 32, 187–196 (2016).
Ranson, H. & Killeen, G. F. Insecticide-resistant malaria vectors must be tackled. Lancet 391, 1551–1552 (2018).
The malERA Consultative Group on Vector Control. A research agenda for malaria eradication: vector control. PLoS Med. 8, e1000401 (2011).
Tiono, A. B. et al. Efficacy of Olyset Duo, a bednet containing pyriproxyfen and permethrin, versus a permethrin-only net against clinical malaria in an area with highly pyrethroid-resistant vectors in rural Burkina Faso: a cluster-randomised controlled trial. Lancet 392, 569–580 (2018).
Bayili, K. et al. Evaluation of efficacy of Interceptor G2, a long-lasting insecticide net coated with a mixture of chlorfenapyr and α-cypermethrin, against pyrethroid resistant Anopheles gambiae s. l. in Burkina Faso. Malar. J. 16, 190 (2017).
Parker, J. E. A. et al. Infrared video tracking of Anopheles gambiae at insecticide-treated bed nets reveals rapid decisive impact after brief localised net contact. Sci. Rep. 5, 13392 (2015).
Parker, J. E. A. et al. Host-seeking activity of a Tanzanian population of Anopheles arabiensis at an insecticide treated bed net. Malar. J. 16, 270 (2017).
Lynd, A. & McCall, P. J. Clustering of host-seeking activity of Anopheles gambiae mosquitoes at the top surface of a human-baited bednet. Malar. J. 12, 267 (2013).
Sutcliffe, S. & Yin, S. Behavioural responses of females of two anopheline mosquito species to human-occupied, insecticide-treated and untreated bed nets. Malar. J. 13, 294 (2014).
McCall, P. J. Mosquito bed net assembly. International patent WO2015063455A1 (2015).
Agossa, F. R. et al. Efficacy of various insecticides recommended for indoor residual spraying: pirimiphos methyl, potential alternative to bendiocarb for pyrethroid resistance management in Benin, West Africa. Trans. R. Soc. Trop. Med. Hyg. 108, 84–91 (2014).
Gnanguenon, V. et al. Malaria vectors resistance to insecticides in Benin: current trends and mechanisms involved. Parasit. Vectors 8, 223 (2015).
Akogbéto, M. et al. Bendiocarb, a potential alternative against pyrethroid resistant Anopheles gambiae in Benin, West Africa. Malar. J. 9, 204 (2010).
Griffin, J. T. et al. Reducing Plasmodium falciparum malaria transmission in Africa: a model-based evaluation of intervention strategies. PLoS Med. 7, e1000324 (2010).
Griffin, J. T. et al. Potential for reduction of burden and local elimination of malaria by reducing Plasmodium falciparum malaria transmission: a mathematical modelling study. Lancet Infect. Dis. 16, 465–472 (2016).
White, M. T. et al. Modelling the impact of vector control interventions on Anopheles gambiae population dynamics. Parasit. Vectors 4, 153 (2011).
Conditions for Deployment of Mosquito Nets Treated with a Pyrethroid and Piperonyl Butoxide (WHO, 2017).
Mahama, T. et al. Effectiveness of permanet in Côte d’Ivoire rural areas and residual activity on a knockdown-resistant strain of Anopheles gambiae. J. Med. Entomol. 44, 498–502 (2017).
Toé, K. H. et al. Do bednets including piperonyl butoxide offer additional protection against populations of Anopheles gambiae s. l. that are highly resistant to pyrethroids? An experimental hut evaluation in Burkina Faso. Med. Vet. Entomol. 32, 407–416 (2018).
Sternberg, E. D., Waite, J. L. & Thomas, M. B. Evaluating the efficacy of biological and conventional insecticides with the new ‘MCD bottle’ bioassay. Malar. J. 13, 499 (2014).
Janko, M. M. et al. Strengthening long-lasting insecticidal nets effectiveness monitoring using retrospective analysis of cross-sectional, population-based surveys across sub-Saharan Africa. Sci. Rep. 20, 17110 (2018).
Paton, D. G. et al. Exposing Anopheles mosquitoes to antimalarials blocks Plasmodium parasite transmission. Nature 567, 239–243 (2019).
Edi, C. V. et al. Multiple-insecticide resistance in Anopheles gambiae mosquitoes, Southern Côte d’Ivoire. Emerg. Infect. Dis. 18, 1508–1511 (2012).
Coetzee, M. et al. Anopheles coluzzii and Anopheles amharicus, new members of the Anopheles gambiae complex. Zootaxa 3619, 246–274 (2013).
Guidelines for Laboratory and Field Testing of Long-lasting Insecticidal Nets (WHO, 2013).
Toé, K. H. et al. Increased pyrethroid resistance in malaria vectors and decreased bed net effectiveness, Burkina Faso. Emerg. Infect. Dis. 20, 1691–1696 (2014).
Griffin, J. T., Ferguson, N. M. & Ghani, A. C. Estimates of the changing age-burden of Plasmodium falciparum malaria disease in sub-Saharan Africa. Nat. Commun. 5, 1–10 (2014).
Griffin, J. T. et al. Gradual acquisition of immunity to severe malaria with increasing exposure. Proc. Royal Soc. B 282, 2657–2657 (2015).
Climate Prediction Center (National Weather Service, accessed 24 March 2016); http://www.cpc.ncep.noaa.gov/products/international/
Winskill, P. et al. Malaria Initiative, Plasmodium falciparum transmission and mortality: a modelling study. PLoS Med. 14, e1002448 (2017).
Angarita-Jaimes, N. et al. A novel video-tracking system to record behaviour of nocturnal mosquitoes attacking human hosts. J. Royal Soc. Interface 13, 20150974 (2016).
Acknowledgements
We are grateful to the community at Tengrela, Burkina Faso, who continue to host our studies at their village. This research was jointly funded by awards from the UK Medical Research Council (MRC) and the UK Department for International Development (DFID) under the MRC/DFID Concordat Agreement (MR/M011941/1 and MRC Confidence in Concept award MC_PC_13069), and the Wellcome Trust (200222/Z/15/Z). We thank M. Bernardi for her help preparing the figures.
Author information
Authors and Affiliations
Contributions
P.J.M. conceived the barrier bednet and designed the study. G.P.D.M. collected most of the experimental data with assistance from N.L., K.H.T., S.N., W.M.G. and G.M.F. D.T. and C.E.T. designed the video-tracking capture and analysis systems, which V.V. and J.E.A.P. built. T.S.C. and E.S.-S. performed malaria impact predictions. J.J. performed barrier design simulations. G.P.D.M. performed statistical analyses with P.J.M. G.P.D.M. and P.J.M. interpreted results with H.R. and G.M.F. P.J.M. wrote the paper with input from G.P.D.M., G.M.F. and other authors. All authors approved the submitted version.
Corresponding author
Ethics declarations
Competing interests
A patent application (WO2015063455A1) that names P.J.M. was filed by LSTM in respect of the barrier bednet, initially in the UK (7 May 2015), but has now entered the Patent Cooperation Treaty process. LSTM has a research agreement with Vestergaard, which provided LLIN materials but had no role in study design, data collection, analysis and interpretation, report writing or publishing. The authors declare no other competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Insecticide susceptibility status of the wild Anopheles gambiae s. l. population at Tengrela, Banfora in Cascades region of Burkina Faso.
Adult female mosquitoes were tested using the WHO tube test. Mortality rates of less than 95% are indicative of resistance.
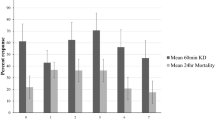
Extended Data Fig. 2 Complete results summary of the hut trial in Tengrela, Cascades Region, Burkina Faso.
Treatment codes: UT (Unmodified untreated polyester bednet), P2 (unmodified Permanet 2.0), P2+P2B (Permanet 2.0 bednet and barrier of P2.0); P2+NPB (P2 net and non-pyrethroid mixture [indoxacarb/ fenazaquin, 3–5%]); P2+OPB (P2 and fenitrothion-dipped barrier, 0.5g/m2); UT+OPB (untreated bednet and fenitrothion-dipped barrier). Outcomes are defined in Methods. Asterisks denote significant differences between treatments (P=0.05-0.01*; 0.01-0.001**;<0.001***). All comparisons vs. UT, unless stated otherwise. Percentage Deterrence: Poisson regression GLM; P2+OPB, n=6, df=5, Z=3.02 P=0.02; UNT+OPB, n=6, df=5, Z=2.21, P= 0.03. Personal protection: Negative Binomial GLM; P2+OPB, n=109, df=5, Z=−2.649, P=0.008. Killing effect: Poisson regression GLM; P2+NPB, n=44, df=5, Z= 2.127, P= 0.03; P2+OPB, n=133, df=5, Z= 7.612, P<0.001; UT+OPB, n=152, df=5, Z=8.320, P<0.001. Percentage exiting: Negative Binomial GLM; UT, n=121, df=5, Z= −5.805 P<0.001. Percentage collected inside net: Negative Binomial GLM; UT, n=163, df=5, Z=−2.047 P<0.0407. Killing effect vs. unmodified P2: Poisson regression GLM; P2+NPB, n=44, df=5, Z= 1.921, P= 0.04; P2+OPB, n=133, df=5, Z= 2.644, P=0.008; UT+OPB, n=152, df=5, Z=5.322, P=0.005. Personal protection vs. unmodified P2: Negative Binomial GLM; P2+OPB, n=109, df=5, Z=1.61, P=0.03.
Extended Data Fig. 3 Transmission model parameter estimates used to test the effect of organophosphate panels on bednets in the Cascades administration region of Burkina Faso.
All other parameters match those previously reported (21,29,30,33). Parameter estimates are noted for: i) standard nets (for example. Permanet 2.0) working optimally; ii) standard nets working as predicted for the resistance scenario where 99% of mosquitoes survive during a discriminatory dose WHO bioassay test in the presence of pyrethroid insecticides; iii) Permanet 2.0 with an organophosphate barrier, and; iv) an untreated net with an organophosphate barrier.
Extended Data Fig. 4 Frequency and duration of mosquito contact with bednets and barriers in the laboratory.
The number, location and duration of mosquito contact at unmodified and barrier bednets; data from video recordings of the bioassays in Fig. 1b (25 female mosquitoes, 1hr). The bednet roof was the primary contact location in all treatments (t-test: IS, P=0.45; IR, P=0.19; IR/OPB, P=0.93). Contact with treated netting (bednet+barrier) was similar between treatments for IS (mean±SD contact/ trial: 959±1032s and 1099±1035s; t-test, P=0.839) and IR mosquitoes (185±144.8 vs. 519±455.7, t-test, P=0.478; Fig. 2g); and between P2 and P2+OPB (185.0±144.8 vs. 212.8±239.1, t-test, P=0.309) or number (249.4±7.2 and 123.5±13; t-test, P=0.056).
Extended Data Fig. 5 Frequency and duration of contact at bednets with OP- treated barriers by wild Anopheles coluzzii in Banfora, Burkina Faso.
The number, location and duration of mosquito contact on barrier bednets recorded during tests (Fig. 1b). Data refer to 2hr video recordings, with 25 female mosquitoes released. Comparisons of number or duration of contacts between treatments were not significant for the bednet or barrier, based on t-tests (normality tested using Shapiro–Wilk test). When bednet and barrier contacts were combined, duration was significantly higher in UT+OPB (t-test; n=5, df=12, t = −2.19, P=0.048).
Extended Data Fig. 6 Behaviour modes of Anopheles gambiae at bednets with or without barriers.
Duration of activity in each behaviour mode; data from video recording of activity of 25 adult female Anopheles gambiae s.l. over 60min (pyrethroid susceptible [IS] or resistant [IR] strains; top) or 120min (wild Burkina Faso population, bottom). Total duration of all tracks classed in each behaviour mode (geometric mean ±SD, seconds). Since multiple mosquitoes were often active simultaneously in the field of view, the total activity times could exceed 60 minutes. Behaviour modes, defined previously12, were as follows: Swooping - tracks that did not contact netting; Visiting - tracks of relatively long flight period interspersed with infrequent bednet contacts, characterized by sharp trajectory turns of ≥80° and 0.4s minimum interval between multiple contacts; Bouncing - tracks of multiple rapid netting contact, at intervals of less than 0.4s, including short flights between contacts, or unbroken contact without being static, for example. ‘walking’ and ‘probing’; Resting - static for at least 0.75 seconds, or velocity less than 1.33mm/s, unbroken contact with net.
Extended Data Fig. 7 Comparison of simulated performances of different barrier designs and heights.
(A) Mean total mosquito population contact time (duration of all contact and resting events; minutes) per experiment for a standard untreated bednet and different untreated barrier designs at different heights. Note: with no negative impact from untreated net contact, virtual mosquitoes revisit the net ad infinitum, hence high contact rates within 1hr. (B) Mean time in minutes to kill the entire mosquito population, when both bednet and barrier are insecticide-treated, by each barrier design and barrier height on treated nets. All net contact areas deliver a dose of 0.05 units per contact. The insecticide treatment is identical on every surface treated, and equivalent to a Permanet 2.0 in terms of repellency. The agent response to contacting a treated net is to decrement health and to select a new random direction and fly away. Thus, the insecticide approximates contact irritancy and not spatial repellency. (C) Mean population kill time when only the barrier is insecticide- treated (dose=1 unit per contact). Note: 5 and 10cm T-barriers did not kill the entire mosquito population in all runs.
Extended Data Fig. 8 Comparing different barrier designs and heights by evaluating performance in silico.
(A) Population kill time (total time needed to achieve complete population death) when insecticide is delivered by both bednet and barrier, for different barrier designs at increasing barrier height. (B) Population kill time as in A, weighted by surface area with a standard unmodified bednet as reference. Plot colours correspond to barrier design borders in Fig. 4.
Supplementary information
Rights and permissions
About this article
Cite this article
Murray, G.P.D., Lissenden, N., Jones, J. et al. Barrier bednets target malaria vectors and expand the range of usable insecticides. Nat Microbiol 5, 40–47 (2020). https://doi.org/10.1038/s41564-019-0607-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41564-019-0607-2
This article is cited by
-
Advanced methods for insect nets: red-colored nets contribute to sustainable agriculture
Scientific Reports (2024)
-
The interplay between malaria vectors and human activity accounts for high residual malaria transmission in a Burkina Faso village with universal ITN coverage
Parasites & Vectors (2023)
-
Insecticidal roof barriers mounted on untreated bed nets can be as effective against Anopheles gambiae as regular insecticide-treated bed nets
Scientific Reports (2023)
-
Quantifying the direct and indirect protection provided by insecticide treated bed nets against malaria
Nature Communications (2023)
-
Malaria & mRNA Vaccines: A Possible Salvation from One of the Most Relevant Infectious Diseases of the Global South
Acta Parasitologica (2023)