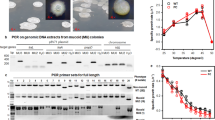
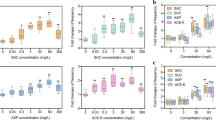
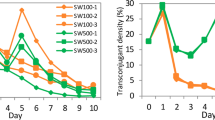
Abstract
Engineering microorganisms to promote human or plant health will require manipulation of robust bacteria that are capable of surviving in harsh, competitive environments. Genetic engineering of undomesticated bacteria can be limited by an inability to transfer DNA into the cell. Here we developed an approach based on the integrative and conjugative element from Bacillus subtilis (ICEBs1) to overcome this problem. A donor strain (XPORT) was built to transfer miniaturized integrative and conjugative elements (mini-ICEBs1) to undomesticated bacteria. The strain was engineered to enable inducible control over conjugation, to integrate delivered DNA into the chromosome of the recipient, to restrict spread of heterologous DNA through separation of the type IV secretion system from the transferred DNA, and to enable simple isolation of engineered bacteria through a d-alanine auxotrophy. Efficient DNA transfer (10–1 to 10–7 conjugation events per donor) is demonstrated using 35 Gram-positive strains isolated from humans (skin and gut) and soil. Mini-ICEBs1 was used to rapidly characterize the performance of an isopropyl-β-d-thiogalactoside (IPTG)-inducible reporter across dozens of strains and to transfer nitrogen fixation to four Bacillus species. Finally, XPORT was introduced to soil to demonstrate DNA transfer under non-ideal conditions.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Head, I. M., Jones, D. M. & Röling, W. F. M. Marine microorganisms make a meal of oil. Nat. Rev. Microbiol. 4, 173–182 (2006).
Keller, L. & Surette, M. G. Communication in bacteria: an ecological and evolutionary perspective. Nat. Rev. Microbiol. 4, 249–258 (2006).
Clardy, J., Fischbach, M. A. & Walsh, C. T. New antibiotics from bacterial natural products. Nat. Biotechnol. 24, 1541–1550 (2006).
Adams, B. L. The next generation of synthetic biology chassis: moving synthetic biology from the laboratory to the field. ACS Synth. Biol. 5, 1328–1330 (2016).
Dubnau, D. DNA uptake in bacteria. Annu. Rev. Microbiol. 53, 217–244 (1999).
Aune, T. E. V. & Aachmann, F. L. Methodologies to increase the transformation efficiencies and the range of bacteria that can be transformed. Appl. Microbiol. Biotechnol. 85, 1301–1313 (2010).
Johnston, C., Martin, B., Fichant, G., Polard, P. & Claverys, J.-P. Bacterial transformation: distribution, shared mechanisms and divergent control. Nat. Rev. Microbiol. 12, 181–196 (2014).
Norman, A., Hansen, L. H. & Sørensen, S. J. Conjugative plasmids: vessels of the communal gene pool. Phil. Trans. R. Soc. B 364, 2275–2289 (2009).
Mazodier, P. & Davies, J. Gene transfer between distantly related bacteria. Annu. Rev. Genet. 25, 147–171 (1991).
Huddleston, J. R. Horizontal gene transfer in the human gastrointestinal tract: potential spread of antibiotic resistance genes. Infect. Drug Resist. 7, 167–176 (2014).
Shoeb, E. et al. Horizontal gene transfer of stress resistance genes through plasmid transport. World J. Microbiol. Biotechnol. 28, 1021–1025 (2012).
Frost, L. S., Leplae, R., Summers, A. O. & Toussaint, A. Mobile genetic elements: the agents of open source evolution. Nat. Rev. Microbiol. 3, 722–732 (2005).
Hoekema, A., Hirsch, P. R., Hooykaas, P. J. J. & Schilperoort, R. A. A binary plant vector strategy based on separation of vir- and T-region of the Agrobacterium tumefaciens Ti-plasmid. Nature 303, 179–180 (1983).
Simon, R., Priefer, U. & Pühler, A. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram negative bacteria. Bio/Technology 1, 784–791 (1983).
Cuív, P. Ó. et al. Isolation of genetically tractable most-wanted bacteria by metaparental mating. Sci. Rep. 5, 13282 (2015).
Henschke, R. B. & Schmidt, F. R. J. Plasmid mobilization from genetically engineered bacteria to members of the indigenous soil microflora in situ. Curr. Microbiol. 20, 105–110 (1990).
Babic, A., Guérout, A.-M. & Mazel, D. Construction of an improved RP4 (RK2)-based conjugative system. Res. Microbiol. 159, 545–549 (2008).
Ferrières, L. et al. Silent mischief: bacteriophage Mu insertions contaminate products of Escherichia coli random mutagenesis performed using suicidal transposon delivery plasmids mobilized by broad-host-range RP4 conjugative machinery. J. Bacteriol. 192, 6418–6427 (2010).
Strand, T. A., Lale, R., Degnes, K. F., Lando, M. & Valla, S. A new and improved host-independent plasmid system for RK2-based conjugal transfer. PLOS ONE 9, e90372 (2014).
Bañuelos-Vazquez, L. A., Tejerizo, G. T. & Brom, S. Regulation of conjugative transfer of plasmids and integrative conjugative elements. Plasmid 91, 82–89 (2017).
Roberts, A. P. & Mullany, P. A modular master on the move: the Tn916 family of mobile genetic elements. Trends Microbiol. 17, 251–258 (2009).
Lee, C. A., Auchtung, J. M., Monson, R. E. & Grossman, A. D. Identification and characterization of int (integrase), xis (excisionase) and chromosomal attachment sites of the integrative and conjugative element ICEBs1 of Bacillus subtilis. Mol. Microbiol. 66, 1356–1369 (2007).
Auchtung, J. M., Lee, C. A., Monson, R. E., Lehman, A. P. & Grossman, A. D. Regulation of a Bacillus subtilis mobile genetic element by intercellular signaling and the global DNA damage response. Proc. Natl Acad. Sci. USA 102, 12554–12559 (2005).
Auchtung, J. M., Lee, C. A., Garrison, K. L. & Grossman, A. D. Identification and characterization of the immunity repressor (ImmR) that controls the mobile genetic element ICEBs1 of Bacillus subtilis. Mol. Microbiol. 64, 1515–1528 (2007).
Auchtung, J. M., Lee, C. A. & Grossman, A. D. Modulation of the ComA-dependent quorum response in Bacillus subtilis by multiple Rap proteins and Phr peptides. J. Bacteriol. 188, 5273–5285 (2006).
Thomas, J., Lee, C. A. & Grossman, A. D. A conserved helicase processivity factor is needed for conjugation and replication of an integrative and conjugative element. PLoS Genet. 9, e1003198 (2013).
Wright, L. D., Johnson, C. M. & Grossman, A. D. Identification of a single strand origin of replication in the integrative and conjugative element ICEBs1 of Bacillus subtilis. PLoS Genet. 11, e1005556 (2015).
Leonetti, C. T. et al. Critical components of the conjugation machinery of the integrative and conjugative element ICEBs1 of Bacillus subtilis. J. Bacteriol. 197, 2558–2567 (2015).
DeWitt, T. & Grossman, A. D. The bifunctional cell wall hydrolase CwlT is needed for conjugation of the integrative and conjugative element ICEBs1 in Bacillus subtilis and B. anthracis. J. Bacteriol. 196, 1588–1596 (2014).
Lee, C. A., Thomas, J. & Grossman, A. D. The Bacillus subtilis conjugative transposon ICEBs1 mobilizes plasmids lacking dedicated mobilization functions. J. Bacteriol. 194, 3165–3172 (2012).
Lee, C. A. & Grossman, A. D. Identification of the origin of transfer (oriT) and DNA relaxase required for conjugation of the integrative and conjugative element ICEBs1 of Bacillus subtilis. J. Bacteriol. 189, 7254–7261 (2007).
Chopra, I. & Roberts, M. Tetracycline antibiotics: mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol. Mol. Biol. Rev. 65, 232–260 (2001).
Horinouchi, S. & Weisblum, B. Nucleotide sequence and functional map of pC194, a plasmid that specifies inducible chloramphenicol resistance. J. Bacteriol. 150, 815–825 (1982).
Bhavsar, A. P., Zhao, X. & Brown, E. D. Development and characterization of a xylose-dependent system for expression of cloned genes in Bacillus subtilis: conditional complementation of a teichoic acid mutant. Appl. Environ. Microbiol. 67, 403–410 (2001).
Wecke, J., Madela, K. & Fischer, W. The absence of d-alanine from lipoteichoic acid and wall teichoic acid alters surface charge, enhances autolysis and increases susceptibility to methicillin in Bacillus subtilis. Microbiology 143, 2953–2960 (1997).
Chung, Y. S. & Dubnau, D. ComC is required for the processing and translocation of comGC, a pilin-like competence protein of Bacillus subtilis. Mol. Microbiol. 15, 543–551 (1995).
Menard, K. L. & Grossman, A. D. Selective pressures to maintain attachment site specificity of integrative and conjugative elements. PLoS Genet. 9, e1003623 (2013).
Lee, C. A., Babic, A. & Grossman, A. D. Autonomous plasmid-like replication of a conjugative transposon. Mol. Microbiol. 75, 268–279 (2010).
Zhu, B. & Stülke, J. SubtiWiki in 2018: from genes and proteins to functional network annotation of the model organism Bacillus subtilis. Nucleic Acids Res. 46, D743–D748 (2018).
Leonhardt, H. & Alonso, J. C. Parameters affecting plasmid stability in Bacillus subtilis. Gene 103, 107–111 (1991).
Cardinale, S., Joachimiak, M. P. & Arkin, A. P. Effects of genetic variation on the E. coli host–circuit interface. Cell Rep. 4, 231–237 (2013).
Smanski, M. J. et al. Functional optimization of gene clusters by combinatorial design and assembly. Nat. Biotechnol. 32, 1241–1249 (2014).
Wang, L. et al. A minimal nitrogen fixation gene cluster from Paenibacillus sp. WLY78 enables expression of active nitrogenase in Escherichia coli. PLoS Genet. 9, e1003865 (2013).
Kushwaha, M. & Salis, H. M. A portable expression resource for engineering cross-species genetic circuits and pathways. Nat. Commun. 6, 7832 (2015).
Vieira, F. C. S. & Nahas, E. Comparison of microbial numbers in soils by using various culture media and temperatures. Microbiol. Res. 160, 197–202 (2005).
Nielsen, A. A. & Voigt, C. A. Multi-input CRISPR/Cas genetic circuits that interface host regulatory networks. Mol. Syst. Biol. 10, 763 (2014).
Wang, K., Neumann, H., Peak-Chew, S. Y. & Chin, J. W. Evolved orthogonal ribosomes enhance the efficiency of synthetic genetic code expansion. Nat. Biotechnol. 25, 770–777 (2007).
Liu, C.C. et al. Toward an orthogonal central dogma. Nat. Chem. Bio. 14, 103–106 (2018).
Dereeper, A. et al. Phylogeny.fr: robust phylogenetic analysis for the non-specialist. Nucleic Acids Res. 36, W465–W469 (2008).
Das, S., Noe, J. C., Paik, S. & Kitten, T. An improved arbitrary primed PCR method for rapid characterization of transposon insertion sites. J. Microbiol. Methods 63, 89–94 (2005).
Stewart, W. D. P., Fitzgerald, G. P. & Burris, R. H. In situ studies on nitrogen fixation with the acetylene reduction technique. Science 158, 536 (1967).
Acknowledgements
We thank E. Alm (Massachusetts Institute of Technology) and R. Xavier (Broad Institute) for the bacterial isolates obtained from human gut and skin. This work was supported by the US Defense Advanced Research Projects Agency’s Biological Robustness in Complex Settings program award (HR0011-15-2-0033), the US Office of the Secretary of Defense Laboratory University Collaborative Initiative fellowship, the Office of the Secretary of Defense Applied Research for the Advancement of S&T Priorities program on Synthetic Biology for Military Environments, and the National Institute of General Medical Sciences (GM041934).
Author information
Authors and Affiliations
Contributions
J.A.N.B., C.A.V. and A.D.G. conceived the study and designed the ICEBs1 engineering and characterization experiments. J.A.N.B. and A.J.T. performed ICEBs1 engineering and characterization experiments and analysed the data. B.L.A. and D.N.S.-C. conceived and designed soil sensor experiments. B.L.A. and R.L.R. performed the soil sensor experiments and analysed the data. J.A.N.B., C.A.V., B.L.A. and R.L.R. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figures 1–15, Supplementary Tables 1–5.
Supplementary File 1
Sequence file for B. subtilis JAB1000 mini-ICEBs1_M1.
Supplementary File 2
Sequence file for B. subtilis JAB447 mini-ICEBs1_M2.
Supplementary File 3
Sequence file for B. subtilis JAB854 mini-ICEBs1_M3.
Supplementary File 4
Sequence file for B. subtilis JAB927 mini-ICEBs1_M4.
Supplementary File 5
Sequence file for B. subtilis JAB932 mini-ICEBs1_M1.
Supplementary File 6
Sequence file for B. subtilis JAB943 mini-ICEBs1_M7.
Supplementary File 7
Sequence file for B. subtilis JAB944 mini-ICEBs1_M6.
Supplementary File 8
Sequence file for B. subtilis JAB951 mini-ICEBs1_M5.
Supplementary File 9
Sequence file for B. subtilis JAB981 mini-ICEBs1_M1.
Supplementary File 10
Sequence file for B. subtilis JH642 ICEBs1.
Supplementary File 11
Sequence file for plasmid pJAB205.
Supplementary File 12
Sequence file for plasmid pJAB273.
Supplementary File 13
Sequence file for plasmid pJAB309.
Supplementary File 14
Sequence file for plasmid pJAB423.
Supplementary File 15
Sequence file for plasmid pJAB463.
Supplementary File 16
Sequence file for plasmid pJAB716.
Supplementary File 17
Sequence file for plasmid pJAB775.
Supplementary File 18
Sequence file for plasmid pJAB778.
Supplementary File 19
Sequence file for plasmid pJAB980.
Supplementary File 20
Sequence file for plasmid pJAB988.
Supplementary File 21
Sequence file for plasmid pJAB-001.
Supplementary File 22
Sequence file for plasmid pJAB-002.
Supplementary File 23
Sequence file for plasmid pJAB-003.
Supplementary File 24
Sequence file for plasmid pJAB-005.
Supplementary File 25
Sequence file for plasmid pJAB- 009.
Supplementary File 26
Sequence file for plasmid pJAB- 017.
Rights and permissions
About this article
Cite this article
Brophy, J.A.N., Triassi, A.J., Adams, B.L. et al. Engineered integrative and conjugative elements for efficient and inducible DNA transfer to undomesticated bacteria. Nat Microbiol 3, 1043–1053 (2018). https://doi.org/10.1038/s41564-018-0216-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41564-018-0216-5
This article is cited by
-
Sentinel cells programmed to respond to environmental DNA including human sequences
Nature Chemical Biology (2024)
-
Experimental warming accelerates positive soil priming in a temperate grassland ecosystem
Nature Communications (2024)
-
Choreographing root architecture and rhizosphere interactions through synthetic biology
Nature Communications (2024)
-
Oxygen mediated mobilization and co-occurrence of antibiotic resistance in lab-scale bioreactor using metagenomic binning
World Journal of Microbiology and Biotechnology (2024)
-
Addressable and adaptable intercellular communication via DNA messaging
Nature Communications (2023)