Abstract
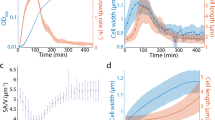
In nature, microorganisms exhibit different volumes spanning six orders of magnitude1. Despite their capability to create different sizes, a clonal population in a given environment maintains a uniform size across individual cells. Recent studies in eukaryotic and bacterial organisms showed that this homogeneity in cell size can be accomplished by growing a constant size between two cell cycle events (that is, the adder model2,3,4,5,6). Demonstration of the adder model led to the hypothesis that this phenomenon is a consequence of convergent evolution. Given that archaeal cells share characteristics with both bacteria and eukaryotes, we investigated whether and how archaeal cells exhibit control over cell size. To this end, we developed a soft-lithography method of growing the archaeal cells to enable quantitative time-lapse imaging and single-cell analysis, which would be useful for other microorganisms. Using this method, we demonstrated that Halobacterium salinarum, a hypersaline-adapted archaeal organism, grows exponentially at the single-cell level and maintains a narrow-size distribution by adding a constant length between cell division events. Interestingly, the archaeal cells exhibited greater variability in cell division placement and exponential growth rate across individual cells in a population relative to those observed in Escherichia coli 6,7,8,9. Here, we present a theoretical framework that explains how these larger fluctuations in archaeal cell cycle events contribute to cell size variability and control.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Deforet, M., van Ditmarsch, D. & Xavier, J. B. Cell-size homeostasis and the incremental rule in a bacterial pathogen. Biophys. J. 109, 521–528 (2015).
Soifer, I., Robert, L. & Amir, A. Single-cell analysis of growth in budding yeast and bacteria reveals a common size regulation strategy. Curr. Biol. 26, 356–361 (2016).
Sauls, J. T., Li, D. & Jun, S. Adder and a coarse-grained approach to cell size homeostasis in bacteria. Curr. Opin. Cell Biol. 38, 38–44 (2016).
Amir, A. Cell size regulation in bacteria. Phys. Rev. Lett. 112, 208102 (2014).
Campos, M. et al. A constant size extension drives bacterial cell size homeostasis. Cell 159, 1433–1446 (2014).
Taheri-Araghi, S. et al. Cell-size control and homeostasis in bacteria. Curr. Biol. 25, 385–391 (2015).
Wang, P. et al. Robust growth of Escherichia coli. Curr. Biol. 20, 1099–1103 (2010).
Godin, M. et al. Using buoyant mass to measure the growth of single cells. Nat. Methods 7, 387–390 (2010).
Guberman, J. M., Fay, A., Dworkin, J., Wingreen, N. S. & Gitai, Z. PSICIC: noise and asymmetry in bacterial division revealed by computational image analysis at sub-pixel resolution. PLoS Comput. Biol. 4, e1000233 (2008).
Young, K. D. The selective value of bacterial shape. Microbiol. Mol. Biol. Rev. 70, 660–703 (2006).
Fievet, A. et al. Single-cell analysis of growth and cell division of the anaerobe Desulfovibrio vulgaris Hildenborough. Front. Microbiol. 6, 1378 (2015).
Iyer-Biswas, S. et al. Scaling laws governing stochastic growth and division of single bacterial cells. Proc. Natl Acad. Sci. USA 111, 15912–15917 (2014).
Banerjee, S. et al. Biphasic growth dynamics control cell division in Caulobacter crescentus. Nat. Microbiol. 2, 17116 (2017).
Nobs, J. B., Maerkl, S. J. & Polymenis, M. Long-term single cell analysis of S. pombe on a microfluidic microchemostat array. PLoS ONE 9, e93466 (2014).
Hawkins, M., Malla, S., Blythe, M. J., Nieduszynski, C. A. & Allers, T. Accelerated growth in the absence of DNA replication origins. Nature 503, 544–547 (2013).
Herrmann, U. & Soppa, J. Cell cycle-dependent expression of an essential SMC-like protein and dynamic chromosome localization in the archaeon Halobacterium salinarum. Mol. Microbiol. 46, 395–409 (2002).
Kelman, L. M. & Kelman, Z. Archaeal DNA replication. Annu. Rev. Genet. 48, 71–97 (2014).
Duggin, I. G. et al. CetZ tubulin-like proteins control archaeal cell shape. Nature 519, 362–365 (2015).
Lindås, A.-C. & Bernander, R. The cell cycle of archaea. Nat. Rev. Microbiol. 11, 627–638 (2013).
Popławski, A. & Bernander, R. Nucleoid structure and distribution in thermophilic archaea. J. Bacteriol. 179, 7625–7630 (1997).
Lundgren, M. & Bernander, R. Genome-wide transcription map of an archaeal cell cycle. Proc. Natl Acad. Sci. USA 104, 2939–2944 (2007).
Zaremba-Niedzwiedzka, K. et al. Asgard archaea illuminate the origin of eukaryotic cellular complexity. Nature 541, 353–358 (2017).
Amir, A. Is cell size a spandrel? eLife 6, e22186 (2017).
Ghusinga, K. R., Vargas-Garcia, C. A. & Singh, A. A mechanistic stochastic framework for regulating bacterial cell division. Sci. Rep. 6, 30229 (2016).
Osella, M., Nugent, E. & Cosentino Lagomarsino, M. Concerted control of Escherichia coli cell division. Proc. Natl Acad. Sci. USA 111, 3431–3435 (2014).
Kennard, A. S. et al. Individuality and universality in the growth-division laws of single E. coli cells. Phys. Rev. E 93, 012408 (2016).
Rego, E. H., Audette, R. E. & Rubin, E. J. Deletion of a mycobacterial divisome factor collapses single-cell phenotypic heterogeneity. Nature 546, 153–157 (2017).
Zheng, H. et al. Interrogating the Escherichia coli cell cycle by cell dimension perturbations. Proc. Natl Acad. Sci. USA 113, 15000–15005 (2016).
Ho, P.-Y. & Amir, A. Simultaneous regulation of cell size and chromosome replication in bacteria. Front. Microbiol. 6, 662 (2015).
Ursell, T. S. et al. Rod-like bacterial shape is maintained by feedback between cell curvature and cytoskeletal localization. Proc. Natl Acad. Sci. USA 111, E1025–E1034 (2014).
Renner, L. D., Eswaramoorthy, P., Ramamurthi, K. S. & Weibel, D. B. Studying biomolecule localization by engineering bacterial cell wall curvature. PLoS ONE 8, e84143 (2013).
Paintdakhi, A. et al. Oufti: an integrated software package for high-accuracy, high-throughput quantitative microscopy analysis. Mol. Microbiol. 99, 767–777 (2016).
Sliusarenko, O., Heinritz, J., Emonet, T. & Jacobs-Wagner, C. High-throughput, subpixel precision analysis of bacterial morphogenesis and intracellular spatio-temporal dynamics. Mol. Microbiol. 80, 612–627 (2011).
Wallden, M., Fange, D., Lundius, E. G., Baltekin, Ö. & Elf, J. The synchronization of replication and division cycles in individual E. coli cells. Cell 166, 729–739 (2016).
Acknowledgements
We thank T. Ursell for Morphometrics, D. B. Weibel for providing a fabrication facility, and K. A. Dulmage, M. Kapoor and W. Marshall for stimulating discussions. This work was supported by a Searle Scholars Award and NIH grant DP2AI117923-01 to E.C.G.; a HHMI Helen Hay Whitney Foundation Fellowship to Y.J.E.; the Harvard MRSEC program of the NSF DMR 14-20570 to P.H.; an A. P. Sloan Foundation grant and a Kavli Foundation grant to A.A.; and an NSF grant MCB-141-7750 and Duke Arts and Sciences Research Council Committee on Faculty Research grant to A.K.S., A.A., L.D.R. and E.C.G. also acknowledge support from the Volkswagen Foundation.
Author information
Authors and Affiliations
Contributions
Y.-J.E., A.S., E.G. and A.A. conceived and designed the experiments. Y.-J.E., M.K., L.D.R. and S.L. performed the experiments. Y.-J.E., P.-Y.H., L.R. and A.A. analysed the data. Y.-J.E., P.-Y.H. and A.A. developed and evaluated the theoretical framework. Y.-J.E., P.-Y.H., L.D.R., L.R., A.S., E.G. and A.A. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Tables 1–4, Supplementary Figures 1–9, Supplementary Notes.
Videos
Supplementary Video 1
Halobacterium salinarum growth time-lapse.
Rights and permissions
About this article
Cite this article
Eun, YJ., Ho, PY., Kim, M. et al. Archaeal cells share common size control with bacteria despite noisier growth and division. Nat Microbiol 3, 148–154 (2018). https://doi.org/10.1038/s41564-017-0082-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41564-017-0082-6
This article is cited by
-
Robust replication initiation from coupled homeostatic mechanisms
Nature Communications (2022)
-
Tracking bacterial lineages in complex and dynamic environments with applications for growth control and persistence
Nature Microbiology (2021)
-
Studying life at the extremes
Nature (2020)
-
Efficient computation of stochastic cell-size transient dynamics
BMC Bioinformatics (2019)
-
Cell size sensing—a one-dimensional solution for a three-dimensional problem?
BMC Biology (2019)