Abstract
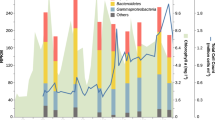
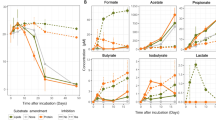
Scientific drilling has identified a biosphere in marine sediments1, which contain many uncultivated microbial groups known only by their DNA sequences2,3,4. Recycling of organic matter in sediments is an important component of biogeochemical cycles because marine sediments are critical for long-term carbon storage5. Turnover of carbon is hypothesized to be driven by the secretion of enzymes by microbial organisms5,6,7, which act to break down macromolecules into constitutive monomers that can be transported into cells. As such, the nature of the microbial secretome often influences the function of a community6. However, the microbial groups involved in this process and the biochemistry they encode is poorly understood. Here, we show that expressed genes from 5 to 159 meters below the seafloor8 (mbsf) encode numerous candidate peptidases and carbohydrate-active enzymes (‘CAZymes’)9 targeted for secretion. The majority (90–99%) were assigned to Bacteria, of which 12% shared the highest sequence similarity with candidate phyla10,11. The remaining putatively secreted proteins shared highest sequence similarity with archaeal and fungal enzymes, which peak in two redox transition zones12. In the shallower redox zone at 30 mbsf, 20% of the transcripts encoding putative secreted peptidases were assigned to lineages7,13,14 of uncultivated Archaea. The target compounds of the predicted secreted proteome show a preference for necromass in the form of microbial cell envelopes as well as plankton and algal detritus. The predicted fungal secreted proteome encodes CAZymes not present in the predicted bacterial or archaeal secreted proteomes, indicating that fungi putatively play a minimal but specialized role in subseafloor carbohydrate recycling.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Kallmeyer, J., Pockalny, R., Adhikari, R., Smith, D. C. & D’Hondt, S. Global distribution of microbial abundance and biomass in subseafloor sediment. Proc. Natl Acad. Sci. USA 109, 16213–16216 (2012).
Fry, J. C., Parkes, R. J., Cragg, B. A., Weightman, A. J. & Webster, G. Prokaryotic biodiversity and activity in the deep subseafloor biosphere. FEMS Microb. Ecol. 66, 181–196 (2008).
Biddle, J. F., Fitz-Gibbon, S., Schuster, S. C., Brenchley, J. E. & House, C. H. Metagenomic signatures of the Peru Margin subseafloor biosphere show a genetically distinct environment. Proc. Natl Acad. Sci. USA 105, 10583–10588 (2008).
Inagaki, F. et al. Biogeographical distribution and diversity of microbes in methane hydrate-bearing deep marine sediments, on the Pacific Ocean Margin. Proc. Natl Acad. Sci. USA 103, 2815–2820 (2006).
Arndt, S. et al. Quantifying the degradation of organic matter in marine sediments: a review and synthesis. Earth Sci. Rev. 123, 53–86 (2013).
Arnosti, C. Microbial extracellular enzymes and the marine carbon cycle. Ann. Rev. Mar. Sci. 3, 401–425 (2011).
Lloyd, K. G. et al. Predominant archaea in marine sediments degrade detrital proteins. Nature 496, 215–218 (2013).
Orsi, W. D., Edgcomb, V. P., Christman, G. D. & Biddle, J. F. Gene expression in the deep biosphere. Nature 499, 205–208 (2013).
Lombard, V., Golaconda Ramulu, H., Drula, E., Coutinho, P. M. & Henrissat, B. The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res. 42, D490–D495 (2014).
Brown, C. T. et al. Unusual biology across a group comprising more than 15% of domain Bacteria. Nature 523, 208–211 (2015).
Rinke, C. et al. Insights into the phylogeny and coding potential of microbial dark matter. Nature 499, 431–437 (2013).
D’Hondt, S. et al. Distributions of microbial activities in deep subseafloor sediments. Science 306, 2216–2221 (2004).
Evans, P. N. et al. Methane metabolism in the archaeal phylum Bathyarchaeota revealed by genome-centric metagenomics. Science 350, 434–438 (2015).
Baker, B. J. et al. Genomic inference of the metabolism of cosmopolitan subsurface Archaea, Hadesarchaea. Nat. Microbiol. 1, 16002 (2016).
Petersen, T. N., Brunak, S., von Heijne, G. & Nielsen, H. SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat. Methods 8, 785–786 (2011).
Overbeek, R. et al. The SEED and the Rapid Annotation of microbial genomes using Subsystems Technology (RAST). Nucleic Acids. Res. 42, D206–D214 (2014).
Wrighton, K. C. et al. Fermentation, hydrogen, and sulfur metabolism in multiple uncultivated bacterial phyla. Science 337, 1661–1665 (2012).
Nobu, M. K. et al. Phylogeny and physiology of candidate phylum ‘Atribacteria’ (OP9/JS1) inferred from cultivation-independent genomics. ISME J. 10, 273–286 (2016).
Hug, L. A. et al. A new view of the tree of life. Nat. Microbiol. 1, 16048 (2016).
Lomstein, B. A., Langerhuus, A. T., D’Hondt, S., Jorgensen, B. B. & Spivack, A. J. Endospore abundance, microbial growth and necromass turnover in deep sub-seafloor sediment. Nature 484, 101–104 (2012).
Canfield, D. E. Factors influencing organic carbon preservation in marine sediments. Chem. Geol. 114, 315–329 (1994).
Ferry, J. G. & Wolfe, R. S. Anaerobic degradation of benzoate to methane by a microbial consortium. Arch. Microbiol. 107, 33–40 (1976).
Vollmer, W., Blanot, D. & de Pedro, M. A. Peptidoglycan structure and architecture. FEMS Microbiol. Rev. 32, 149–167 (2008).
Lazar, C. S. et al. Genomic evidence for distinct carbon substrate preferences and ecological niches of Bathyarchaeota in estuarine sediments. Environ Microbiol. 18, 1200–1211 (2016).
Artzi, L., Bayer, E. A. & Morais, S. Cellulosomes: bacterial nanomachines for dismantling plant polysaccharides. Nat. Rev. Microbiol. 15, 83–95 (2017).
Orsi, W., Biddle, J. F. & Edgcomb, V. Deep sequencing of subseafloor eukaryotic rRNA reveals active Fungi across marine subsurface provinces. PloS ONE 8, e56335 (2013).
Visweswaran, G. R., Dijkstra, B. W. & Kok, J. Murein and pseudomurein cell wall binding domains of bacteria and archaea-a comparative view. Appl. Microbiol. Biotechnol. 92, 921–928 (2011).
Hasegawa, S. & Nordin, J. H. Enzymes that hydrolyze fungal cell wall polysaccharides. I. Purification and properties of an endo-α-d-(1–3)-glucanase from Trichoderma. J Biol. Chem. 244, 5460–5470 (1969).
Rho, M., Tang, H. & Ye, Y. FragGeneScan: predicting genes in short and error-prone reads. Nucleic Acids Res. 38, e191 (2010).
Hansen, G. & Hilgenfeld, R. Architechture and regulation of HtrA-family proteins involved in protein quality control and stress response. Cell. Mol. Life Sci. 70, 761–765 (2012).
Acknowledgements
This work was supported by the Deutsche Forschungsgemeinschaft (DFG) through Project OR 417/1-1 (W.D.O.), and also by Ludwig-Maximilians Universität München Junior Researcher Fund (W.D.O.). T.A.R. is supported by a Royal Society University Research Fellowship. We thank O. Voigt (LMU Munich) for his help in installing the stand-alone (command line) implementation of SignalP.
Author information
Authors and Affiliations
Contributions
W.D.O. and T.A.R. conceived the idea for the study, W.D.O. wrote the paper; W.D.O. and W.R.F. analysed data, W.R.F. developed analytical tools. All authors participated in data interpretation and provided editorial comments on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary Information
Supplementary Tables 1 and 2, Supplementary Figure 1.
Rights and permissions
About this article
Cite this article
Orsi, W.D., Richards, T.A. & Francis, W.R. Predicted microbial secretomes and their target substrates in marine sediment. Nat Microbiol 3, 32–37 (2018). https://doi.org/10.1038/s41564-017-0047-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41564-017-0047-9
This article is cited by
-
Metagenomic analysis of carbohydrate-active enzymes and their contribution to marine sediment biodiversity
World Journal of Microbiology and Biotechnology (2024)
-
The majority of microorganisms in gas hydrate-bearing subseafloor sediments ferment macromolecules
Microbiome (2023)
-
High Microeukaryotic Diversity in the Cold-Seep Sediment
Microbial Ecology (2023)
-
Multi-genome metabolic modeling predicts functional inter-dependencies in the Arabidopsis root microbiome
Microbiome (2022)
-
Ecogenomics sheds light on diverse lifestyle strategies in freshwater CPR
Microbiome (2022)