Abstract
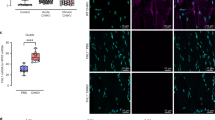
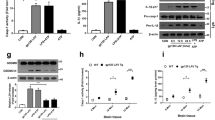
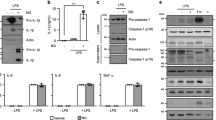
Mosquito-borne viruses can cause severe inflammatory diseases and there are limited therapeutic solutions targeted specifically at virus-induced inflammation. Chikungunya virus (CHIKV), a re-emerging alphavirus responsible for several outbreaks worldwide in the past decade, causes debilitating joint inflammation and severe pain. Here, we show that CHIKV infection activates the NLRP3 inflammasome in humans and mice. Peripheral blood mononuclear cells isolated from CHIKV-infected patients showed elevated NLRP3, caspase-1 and interleukin-18 messenger RNA expression and, using a mouse model of CHIKV infection, we found that high NLRP3 expression was associated with peak inflammatory symptoms. Inhibition of NLRP3 activation using the small-molecule inhibitor MCC950 resulted in reduced CHIKV-induced inflammation and abrogated osteoclastogenic bone loss and myositis, but did not affect in vivo viral replication. Mice treated with MCC950 displayed lower expression levels of the cytokines interleukin-6, chemokine ligand 2 and tumour necrosis factor in joint tissue. Interestingly, MCC950 treatment abrogated disease signs in mice infected with a related arthritogenic alphavirus, Ross River virus, but not in mice infected with West Nile virus—a flavivirus. Here, using mouse models of alphavirus-induced musculoskeletal disease, we demonstrate that NLRP3 inhibition in vivo can reduce inflammatory pathology and that further development of therapeutic solutions targeting inflammasome function could help treat arboviral diseases.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Guo, H., Callaway, J. B. & Ting, J. P.-Y. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat. Med. 21, 677–687 (2015).
Finlay, B. B. & McFadden, G. Anti-immunology: evasion of the host immune system by bacterial and viral pathogens. Cell 124, 767–782 (2006).
Kanneganti, T.-D. Central roles of NLRs and inflammasomes in viral infection. Nat. Rev. Immunol. 10, 688–698 (2010).
Morrison, T. E. et al. Characterization of Ross River virus tropism and virus-induced inflammation in a mouse model of viral arthritis and myositis. J. Virol. 80, 737–749 (2005).
Ekchariyawat, P. et al. Inflammasome signaling pathways exert antiviral effect against chikungunya virus in human dermal fibroblasts. Infect. Genet. Evol. 32, 401–408 (2015).
McAuley, J. L. et al. Activation of the NLRP3 inflammasome by IAV virulence protein PB1-F2 contributes to severe pathophysiology and disease. PLoS Pathog. 9, e1003392 (2013).
Chakrabarti, A. et al. RNase L activates the NLRP3 inflammasome during viral infections. Cell Host Microbe 17, 466–477 (2015).
Ichinohe, T., Lee, H. K., Ogura, Y., Flavell, R. & Iwasaki, A. Inflammasome recognition of influenza virus is essential for adaptive immune responses. J. Exp. Med. 206, 79–87 (2009).
Negash, A. A. et al. IL-1β production through the NLRP3 inflammasome by hepatic macrophages links hepatitis C virus infection with liver inflammation and disease. PloS Pathog. 9, e1003330 (2013).
Johnson, K. E., Chikoti, L. & Chandran, B. Herpes simplex virus 1 infection induces activation and subsequent inhibition of the IFI16 and NLRP3 inflammasomes. J. Virol. 87, 5005–5018 (2013).
Getts, D. R. et al. Therapeutic inflammatory monocyte modulation using immune-modifying microparticles. Sci. Transl. Med. 6, 219ra7 (2014).
Griffin, D. E. Alphaviruses. Encyclopedia Of Molecular Medicine (John Wiley & Sons, Hoboken, NJ, 2002).
Suhrbier, A., Jaffar-Bandjee, M.-C. & Gasque, P. Arthritogenic alphaviruses—an overview. Nat. Rev. Rheumatol. 8, 420–429 (2012).
Suhrbier, A. & La Linn, M. Clinical and pathologic aspects of arthritis due to Ross River virus and other alphaviruses. Curr. Opin. Rheumatol. 16, 374–379 (2004).
Manimunda, S. P. et al. Clinical progression of chikungunya fever during acute and chronic arthritic stages and the changes in joint morphology as revealed by imaging. Trans. R. Soc. Trop. Med. Hyg. 104, 392–399 (2010).
Chen, I.-Y. & Ichinohe, T. Response of host inflammasomes to viral infection. Trends Microbiol. 23, 55–63 (2015).
Cao-Lormeau, V.-M. & Musso, D. Emerging arboviruses in the Pacific. Lancet 384, 1571–1572 (2014).
Chen, W. et al. Arthritogenic alphaviruses: new insights into arthritis and bone pathology. Trends Microbiol. 23, 35–43 (2015).
Coll, R. C. et al. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat. Med. 21, 248–255 (2015).
Taylor, A., Herrero, L. J., Rudd, P. A. & Mahalingam, S. Mouse models of alphavirus-induced inflammatory disease. J. Gen. Virol. 96, 221–238 (2015).
Kaushik, D. K., Gupta, M., Kumawat, K. L. & Basu, A. NLRP3 inflammasome: key mediator of neuroinflammation in murine Japanese encephalitis. PLoS ONE 7, e32270 (2012).
Nakaya, H. I. et al. Gene profiling of chikungunya virus arthritis in a mouse model reveals significant overlap with rheumatoid arthritis. Arthritis Rheum. 64, 3553–3563 (2012).
Ryman, K. D. & Klimstra, W. B. Closing the gap between viral and noninfectious arthritis. Proc. Natl Acad. Sci. USA 111, 5767–5768 (2014).
Chen, W. et al. Arthritogenic alphaviral infection perturbs osteoblast function and triggers pathologic bone loss. Proc. Natl Acad. Sci. USA 111, 6040–6045 (2014).
Xing, L., Schwarz, E. M. & Boyce, B. F. Osteoclast precursors, RANKL/RANK, and immunology. Immunol. Rev. 208, 19–29 (2005).
Cao, Y. et al. Osterix, a transcription factor for osteoblast differentiation, mediates antitumor activity in murine osteosarcoma. Cancer Res. 65, 1124–1128 (2005).
Pinzone, J. J. et al. The role of dickkopf-1 in bone development, homeostasis, and disease. Blood 113, 517–525 (2009).
Zou, L. et al. Use of RUNX2 expression to identify osteogenic progenitor cells derived from human embryonic stem cells. Stem Cell Reports 4, 190–198 (2015).
Gubler, D. J. Human arbovirus infections worldwide. Ann. NY Acad. Sci 951, 13–24 (2001).
Getts, D. R. et al. Ly6c+ ‘inflammatory monocytes’ are microglial precursors recruited in a pathogenic manner in West Nile virus encephalitis. J. Exp. Med. 205, 2319–2337 (2008).
Weaver, S. C. & Barrett, A. D. T. Transmission cycles, host range, evolution and emergence of arboviral disease. Nat. Rev. Microbiol. 2, 789–801 (2004).
Musso, D. & Gubler, D. J. Zika virus. Clin. Microbiol. Rev. 29, 487–524 (2016).
Shepard, D. S. Cost and burden of dengue and chikungunya from the Americas to Asia. Dengue Bull. 34, 1–5 (2010).
Dubrulle, M., Mousson, L., Moutailler, S., Vazeille, M. & Failloux, A.-B. Chikungunya virus and Aedes mosquitoes: saliva is infectious as soon as two days after oral infection. PloS ONE 4, e5895 (2009).
Styer, L. M. et al. Mosquitoes inoculate high doses of West Nile virus as they probe and feed on live hosts. PloS Pathog. 3, 1262–1270 (2007).
Chen, W. et al. Bindarit, an inhibitor of monocyte chemotactic protein synthesis, protects against bone loss induced by chikungunya virus infection. J. Virol. 89, 581–593 (2015).
Chen, W., Foo, S.-S., Li, R. W., Smith, P. N. & Mahalingam, S. Osteoblasts from osteoarthritis patients show enhanced susceptibility to Ross River virus infection associated with delayed type I interferon responses. Virol. J. 11, 189 (2014).
Enomoto, H. et al. Induction of osteoclast differentiation by Runx2 through receptor activator of nuclear factor-kappa B ligand (RANKL) and osteoprotegerin regulation and partial rescue of osteoclastogenesis in Runx2–/– mice by RANKL transgene. J. Biol. Chem. 278, 23971–23977 (2003).
Poo, Y.-S. et al. Multiple immune factors are involved in controlling acute and chronic chikungunya virus infection. PLoS Negl. Trop. Dis. 8, e3354 (2014).
Rulli, N. E. et al. Amelioration of alphavirus-induced arthritis and myositis in a mouse model by treatment with bindarit, an inhibitor of monocyte chemotactic proteins. Arthritis Rheum. 60, 2513–2523 (2009).
Rulli, N. E. et al. Protection from arthritis and myositis in a mouse model of acute chikungunya virus disease by bindarit, an inhibitor of monocyte chemotactic protein-1 synthesis. J. Infect. Dis 204, 1026–1030 (2011).
Poo, Y.-S. et al. CCR2 deficiency promotes exacerbated chronic erosive neutrophil-dominated chikungunya virus arthritis. J. Virol. 88, 6862–6872 (2014).
Seregin, S. S. et al. NLRP6 function in inflammatory monocytes reduces susceptibility to chemically induced intestinal injury. Mucosal. Immunol. 10, 434–445 (2017).
Ramos, H. J. et al. IL-1β signaling promotes CNS-intrinsic immune control of West Nile virus infection. PLoS Pathog. 8, e1003039 (2012).
Vande Walle, L. et al. Negative regulation of the NLRP3 inflammasome by A20 protects against arthritis. Nature 512, 69–73 (2014).
Bonar, S. L. et al. Constitutively activated NLRP3 inflammasome causes inflammation and abnormal skeletal development in mice. PLoS ONE 7, e35979 (2012).
Gurung, P., Burton, A. & Kanneganti, T.-D. NLRP3 inflammasome plays a redundant role with caspase 8 to promote IL-1β-mediated osteomyelitis. Proc. Natl Acad. Sci. USA 113, 4452–4457 (2016).
Qu, C. et al. NLRP3 mediates osteolysis through inflammation-dependent and -independent mechanisms. FASEB J. 29, 1269–1279 (2015).
Wikan, N. et al. Comprehensive proteomic analysis of white blood cells from chikungunya fever patients of different severities. J. Transl. Med. 12, 96 (2014).
Chow, A. et al. Persistent arthralgia induced by chikungunya virus infection is associated with interleukin-6 and granulocyte macrophage colony-stimulating factor. J. Infect. Dis 203, 149–157 (2011).
Gardner, J. et al. Chikungunya virus arthritis in adult wild-type mice. J. Virol. 84, 8021–8032 (2010).
Dempster, D. W. et al. Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J. Bone Miner. Res. 28, 2–17 (2013).
Acknowledgements
We thank S. Masters (the Walter and Eliza Hall Institute of Medical Research) for kindly providing the caspase-1–/– and ASC–/– mice. This study was supported by grants from the Australian National Health and Medical Research Council (NHMRC) to S.M. (APP1079086). S.M. is the recipient of an NHMRC Senior Research Fellowship (APP1059167) and A.S. is the recipient of an NHMRC Principal Research Fellowship (APP1058391).
Author information
Authors and Affiliations
Contributions
W.C., L.F.P.N. and S.M. conceptualized and designed the study. W.C., S-S.F., A.Z., S.W., L.J.H., K.T., A.T. and J.R.F. performed the mouse CHIKV and RRV experiments and analysed the data. L.D.V. and C.v.V. performed the mouse WNV experiments and analysed the data. R.W.L. performed the bone computed tomography experiments and analysed the data. T.M.W. and R.G. performed MCC950 pharmacokinetics experiments and analysed the data. D.M.O., H.I.N., T-D.K., L.A.J.O., A.A.B.R., N.J.K., A.S. and M.A.C. provided the materials and analysis methods. A.Z. wrote the manuscript. A.Z., R.W.L., N.J.K., A.S., L.F.P.N., M.A.C. and S.M. reviewed and revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary Information
Supplementary Figures 1–7, Supplementary Table 1, Supplementary References.
Rights and permissions
About this article
Cite this article
Chen, W., Foo, SS., Zaid, A. et al. Specific inhibition of NLRP3 in chikungunya disease reveals a role for inflammasomes in alphavirus-induced inflammation. Nat Microbiol 2, 1435–1445 (2017). https://doi.org/10.1038/s41564-017-0015-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41564-017-0015-4
This article is cited by
-
Mechanistic insights into bone remodelling dysregulation by human viral pathogens
Nature Microbiology (2024)
-
Chikungunya fever
Nature Reviews Disease Primers (2023)
-
MAVS signaling is required for preventing persistent chikungunya heart infection and chronic vascular tissue inflammation
Nature Communications (2023)
-
Morphine and Fentanyl Repeated Administration Induces Different Levels of NLRP3-Dependent Pyroptosis in the Dorsal Raphe Nucleus of Male Rats via Cell-Specific Activation of TLR4 and Opioid Receptors
Cellular and Molecular Neurobiology (2022)
-
Blockade of the NLRP3/caspase-1 axis attenuates ketamine-induced hippocampus pyroptosis and cognitive impairment in neonatal rats
Journal of Neuroinflammation (2021)