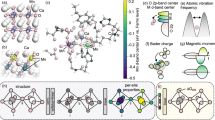
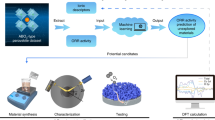
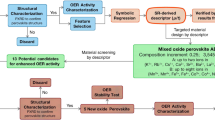
Abstract
Multi-metal oxides in general and perovskite oxides in particular have attracted considerable attention as oxygen evolution electrocatalysts. Although numerous theoretical studies have been undertaken, the most promising perovskite-based catalysts continue to emerge from human-driven experimental campaigns rather than data-driven machine learning protocols, which are often limited by the scarcity of experimental data on which to train the models. This work promises to break this impasse by demonstrating that active learning on even small datasets—but supplemented by informative structural-characterization data and coupled with closed-loop experimentation—can yield materials of outstanding performance. The model we develop not only reproduces several non-obvious and actively studied experimental trends but also identifies a composition of a perovskite oxide electrocatalyst exhibiting an intrinsic overpotential at 10 mA cm–2oxide of 391 mV, which is among the lowest known of four-metal perovskite oxides.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data underlying this work are included in the main text and the Supplementary Information.
Code availability
The comprehensive details concerning the codes used in this study to execute the ML-based protocols can be accessed at the GitHub repository: https://github.com/JunseokMoon/OER2022.
References
Park, S., Shao, Y., Liu, J. & Wang, Y. Oxygen electrocatalysts for water electrolyzers and reversible fuel cells: status and perspective. Energy Environ. Sci. 5, 9331–9344 (2012).
Suen, N.-T. et al. Electrocatalysis for the oxygen evolution reaction: recent development and future perspectives. Chem. Soc. Rev. 46, 337–365 (2017).
Stamenkovic, V. R., Strmcnik, D., Lopes, P. P. & Markovic, N. M. Energy and fuels from electrochemical interfaces. Nat. Mater. 16, 57–69 (2017).
Song, F. et al. Transition metal oxides as electrocatalysts for the oxygen evolution reaction in alkaline solutions: an application-inspired renaissance. J. Am. Chem. Soc. 140, 7748–7759 (2018).
Wang, H.-F., Chen, L., Pang, H., Kaskel, S. & Xu, Q. MOF-derived electrocatalysts for oxygen reduction, oxygen evolution and hydrogen evolution reactions. Chem. Soc. Rev. 49, 1414–1448 (2020).
Bullock, R. M. et al. Using nature’s blueprint to expand catalysis with Earth-abundant metals. Science 369, eabc3183 (2020).
Liu, D. et al. Development of perovskite oxide‐based electrocatalysts for oxygen evolution reaction. Small 17, 2101605 (2021).
Grimaud, A. et al. Activating lattice oxygen redox reactions in metal oxides to catalyse oxygen evolution. Nat. Chem. 9, 457–465 (2017).
Fabbri, E. et al. Dynamic surface self-reconstruction is the key of highly active perovskite nano-electrocatalysts for water splitting. Nat. Mater. 16, 925–931 (2017).
Nørskov, J. K., Bligaard, T., Rossmeisl, J. & Christensen, C. H. Towards the computational design of solid catalysts. Nat. Chem. 1, 37–46 (2009).
Seh, Z. W. et al. Combining theory and experiment in electrocatalysis: insights into materials design. Science 355, eaad4998 (2017).
Weng, B. et al. Simple descriptor derived from symbolic regression accelerating the discovery of new perovskite catalysts. Nat. Commun. 11, 3513 (2020).
Tao, Q., Xu, P., Li, M. & Lu, W. Machine learning for perovskite materials design and discovery. npj Comput. Mater. 7, 23 (2021).
Zahrt, A. F. et al. Prediction of higher-selectivity catalysts by computer-driven workflow and machine learning. Science 363, eaau5631 (2019).
Gensch, T. et al. A comprehensive discovery platform for organophosphorus ligands for catalysis. J. Am. Chem. Soc. 144, 1205–1217 (2022).
Reid, J. P. & Sigman, M. S. Holistic prediction of enantioselectivity in asymmetric catalysis. Nature 571, 343–348 (2019).
Tran, K. & Ulissi, Z. W. Active learning across intermetallics to guide discovery of electrocatalysts for CO2 reduction and H2 evolution. Nat. Catal. 1, 696–703 (2018).
Zhong, M. et al. Accelerated discovery of CO2 electrocatalysts using active machine learning. Nature 581, 178–183 (2020).
Liu, Z. et al. Machine learning with knowledge constraints for process optimization of open-air perovskite solar cell manufacturing. Joule 6, 834–849 (2022).
Coley, C. W. et al. A robotic platform for flow synthesis of organic compounds informed by AI planning. Science 365, eaax1566 (2019).
Mikulak-Klucznik, B. et al. Computational planning of the synthesis of complex natural products. Nature 588, 83–88 (2020).
Shields, B. J. et al. Bayesian reaction optimization as a tool for chemical synthesis. Nature 590, 89–96 (2021).
Angello, N. H. et al. Closed-loop optimization of general reaction conditions for heteroaryl Suzuki-Miyaura coupling. Science 378, 399–405 (2022).
Ekins, S. et al. Exploiting machine learning for end-to-end drug discovery and development. Nat. Mater. 18, 435–441 (2019).
Wang, X. et al. First-principles based machine learning study of oxygen evolution reactions of perovskite oxides using a surface center-environment feature model. Appl. Surf. Sci. 531, 147323 (2020).
Wu, L., Guo, T. & Li, T. Machine learning-accelerated prediction of overpotential of oxygen evolution reaction of single-atom catalysts. iScience 24, 102398 (2021).
Vamathevan, J. et al. Applications of machine learning in drug discovery and development. Nat. Rev. Drug Discov. 18, 463–477 (2019).
Toyao, T. et al. Machine learning for catalysis informatics: recent applications and prospects. ACS Catal. 10, 2260–2297 (2020).
Burke, K. Perspective on density functional theory. J. Chem. Phys. 136, 150901 (2012).
Li, Z., Achenie, L. E. K. & Xin, H. An adaptive machine learning strategy for accelerating discovery of perovskite electrocatalysts. ACS Catal. 10, 4377–4384 (2020).
Shao, Z. & Haile, S. M. A high-performance cathode for the next generation of solid-oxide fuel cells. Nature 431, 170–173 (2004).
McCrory, C. C. L., Jung, S., Peters, J. C. & Jaramillo, T. F. Benchmarking heterogeneous electrocatalysts for the oxygen evolution reaction. J. Am. Chem. Soc. 135, 16977–16987 (2013).
Suntivich, J., May, K. J., Gasteiger, H. A., Goodenough, J. B. & Shao-Horn, Y. A perovskite oxide optimized for oxygen evolution catalysis from molecular orbital principles. Science 334, 1383–1385 (2011).
Beker, W., Gajewska, E. P., Badowski, T. & Grzybowski, B. A. Prediction of major regio‐, site‐, and diastereoisomers in Diels–Alder reactions by using machine‐learning: the importance of physically meaningful descriptors. Angew. Chem. Int. Ed. 58, 4515–4519 (2019).
Zhang, G., Liu, G., Wang, L. & Irvine, J. T. S. Inorganic perovskite photocatalysts for solar energy utilization. Chem. Soc. Rev. 45, 5951–5984 (2016).
Tong, Y. et al. Spin-state regulation of perovskite cobaltite to realize enhanced oxygen evolution activity. Chem 3, 812–821 (2017).
Zhao, X., Gu, F., Wang, Y., Peng, Z. & Liu, J. Surface electronegativity as an activity descriptor to screen oxygen evolution reaction catalysts of Li–O2 battery. ACS Appl. Mater. Interf. 12, 27166–27175 (2020).
Hong, W. T., Welsch, R. E. & Shao-Horn, Y. Descriptors of oxygen-evolution activity for oxides: a statistical evaluation. J. Phys. Chem. C 120, 78–86 (2016).
Han, Z. K. et al. Single-atom alloy catalysts designed by first-principles calculations and artificial intelligence. Nat. Commun. 12, 1833 (2021).
Jiang, X., Wang, Y., Jia, B., Qu, X. & Qin, M. Using machine learning to predict oxygen evolution activity for transition metal hydroxide electrocatalysts. ACS Appl. Mater. Interf. 14, 41141–41148 (2022).
Nguyen, T. X., Liao, Y., Lin, C., Su, Y. & Ting, J. Advanced high entropy perovskite oxide electrocatalyst for oxygen evolution reaction. Adv. Funct. Mater. 31, 2101632 (2021).
Bockris, J. O. & Otagawa, T. Mechanism of oxygen evolution on perovskites. J. Phys. Chem. 87, 2960–2971 (1983).
Yamada, I. et al. Enhanced catalytic activity and stability of the oxygen evolution reaction on tetravalent mixed metal oxide. Chem. Mater. 32, 3893–3903 (2020).
Zhao, B. et al. A tailored double perovskite nanofiber catalyst enables ultrafast oxygen evolution. Nat. Commun. 8, 14586 (2017).
Zhu, Y. et al. Improving the activity for oxygen evolution reaction by tailoring oxygen defects in double perovskite oxides. Adv. Funct. Mater. 29, 1901783 (2019).
Lu, M. et al. Artificially steering electrocatalytic oxygen evolution reaction mechanism by regulating oxygen defect contents in perovskites. Sci. Adv. 8, eabq3563 (2022).
Wu, T. et al. Iron-facilitated dynamic active-site generation on spinel CoAl2O4 with self-termination of surface reconstruction for water oxidation. Nat. Catal. 2, 763–772 (2019).
Rudin, C. Stop explaining black box machine learning models for high stakes decisions and use interpretable models instead. Nat. Mach. Intell. 1, 206–215 (2019).
Li, W., Jacobs, R. & Morgan, D. Predicting the thermodynamic stability of perovskite oxides using machine learning models. Comput. Mater. Sci. 150, 454–463 (2018).
Moskal, M., Beker, W., Szymkuć, S. & Grzybowski, B. A. Scaffold‐directed face selectivity machine‐learned from vectors of non‐covalent interactions. Angew. Chem. Int. Ed. 60, 15230–15235 (2021).
Acknowledgements
This research was supported by the Institute for Basic Science, Korea (project codes IBS-R006-D1 to T.H. and IBS-R020-D1 to B.A.G.) as well as by the Allchemy, Inc. funds (to W.B.). We thank the Korea Basic Science Institute (KBSi) at Busan Center for XPS measurements, the National Instrumentation Center for Environmental Management (NICEM) at Seoul National University for ICP-AES analysis and the National Center for Inter-university Research Facilities (NCIRF) at Seoul National University for high-resolution TEM analysis.
Author information
Authors and Affiliations
Contributions
J.M. designed the workflow and algorithm, collected and analysed data and performed the materials characterization and electrochemical measurements. W.B. supervised the ML and data analysis part of the study and reviewed the algorithm. M.S. participated in writing and technical editing of the manuscript and prepared figures. J.K. participated in algorithm design and supervised the materials characterization. H.S.L. supervised the electrochemical measurements. B.A.G. and T.H. conceived and supervised the research. J.M., B.A.G. and T.H. wrote the manuscript with help from the other authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Materials thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Sections 1–9, Figs. 1–24, Tables 1–15 and references.
Supplementary Data 1
Supplementary dataset including the average predicted overpotential, prediction uncertainty, entropy and A-site electronegativity values for the 10,101 candidate materials.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Moon, J., Beker, W., Siek, M. et al. Active learning guides discovery of a champion four-metal perovskite oxide for oxygen evolution electrocatalysis. Nat. Mater. 23, 108–115 (2024). https://doi.org/10.1038/s41563-023-01707-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41563-023-01707-w
This article is cited by
-
Embracing data science in catalysis research
Nature Catalysis (2024)