Abstract
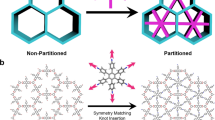
Covalent organic frameworks (COFs) are emerging crystalline porous polymers, showing great potential for applications but lacking gas-triggered flexibility. Atropisomerism was experimentally discovered in 1922 but has rarely been found in crystals with infinite framework structures. Here we report atropisomerism in COF single crystals. The obtained COF atropisomers, namely COF-320 and COF-320-A, have identical chemical and interpenetrated structures but differ in the spatial arrangement of repeating units. In contrast to the rigid COF-320 structure, its atropisomer (COF-320-A) exhibits unconventional gas sorption behaviours with one or more sorption steps in isotherms at different temperatures. Single-crystal structures determined from continuous rotation electron diffraction and in situ powder X-ray diffraction demonstrate that these adsorption steps originate from internal pore expansion with or without changing the crystal space group. COF-320-A recognizes different gases by expanding its internal pores continuously (crystal-to-amorphous transition) or discontinuously (crystal-to-crystal transition) or having mixed transition styles, distinguishing COF-320-A from existing soft/flexible porous crystals. These findings extend atropisomerism from molecules to crystals and propel COFs into the covalently linked soft porous crystal regime, further advancing applications of soft porous crystals in gas sorption, separation and storage.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data are available in the main text or the Supplementary Information. The crystallographic data for single-crystal COF-320-A and COF-320-A2 have been deposited at the Cambridge Crystallographic Data Centre (CCDC, free of charge at https://www.ccdc.cam.ac.uk) under deposition numbers CCDC 2111901 and 2111902, respectively. Correspondence and requests for materials should be addressed to the corresponding authors.
References
Horike, S., Shimomura, S. & Kitagawa, S. Soft porous crystals. Nat. Chem. 1, 695–704 (2009).
Schneemann, A. et al. Flexible metal–organic frameworks. Chem. Soc. Rev. 43, 6062–6096 (2014).
Hiraide, S. et al. High-throughput gas separation by flexible metal–organic frameworks with fast gating and thermal management capabilities. Nat. Commun. 11, 3867 (2020).
Mason, J. A. et al. Methane storage in flexible metal–organic frameworks with intrinsic thermal management. Nature 527, 357–361 (2015).
Zhou, H.-C. “Joe” & Kitagawa, S. Metal–organic frameworks (MOFs). Chem. Soc. Rev. 43, 5415–5418 (2014).
Furukawa, H., Cordova, K. E., O’Keeffe, M. & Yaghi, O. M. The chemistry and applications of metal-organic frameworks. Science 341, 12304444 (2013).
Yaghi, O. M. et al. Reticular synthesis and the design of new materials. Nature 423, 705–714 (2003).
Xu, W. et al. Anisotropic reticular chemistry. Nat. Rev. Mater. 5, 764–779 (2020).
Li, Y., Chen, W., Xing, G., Jiang, D. & Chen, L. New synthetic strategies toward covalent organic frameworks. Chem. Soc. Rev. 49, 2852–2868 (2020).
Krause, S., Hosono, N. & Kitagawa, S. Chemistry of soft porous crystals: structural dynamics and gas adsorption properties. Angew. Chem. Int. Ed. 59, 15325–15341 (2020).
Ortiz, A. U., Boutin, A., Fuchs, A. H. & Coudert, F.-X. Anisotropic elastic properties of flexible metal-organic frameworks: how soft are soft porous crystals? Phys. Rev. Lett. 109, 195502 (2012).
Atkins, P. W. & Shriver, D. F. Shriver & Atkins Inorganic Chemistry 4th edn (W.H. Freeman and Co., 2006).
Ding, M., Cai, X. & Jiang, H.-L. Improving MOF stability: approaches and applications. Chem. Sci. 10, 10209–10230 (2019).
Guan, X., Chen, F., Fang, Q. & Qiu, S. Design and applications of three dimensional covalent organic frameworks. Chem. Soc. Rev. 49, 1357–1384 (2020).
Feng, X., Ding, X. & Jiang, D. Covalent organic frameworks. Chem. Soc. Rev. 41, 6010–6022 (2012).
Chen, Y. et al. Guest-dependent dynamics in a 3D covalent organic framework. J. Am. Chem. Soc. 141, 3298–3303 (2019).
Liu, X. et al. A crystalline three-dimensional covalent organic framework with flexible building blocks. J. Am. Chem. Soc. 143, 2123–2129 (2021).
Zhu, Q. et al. 3D cage COFs: a dynamic three-dimensional covalent organic framework with high-connectivity organic cage nodes. J. Am. Chem. Soc. 142, 16842–16848 (2020).
Cheng, J. K., Xiang, S.-H., Li, S., Ye, L. & Tan, B. Recent advances in catalytic asymmetric construction of atropisomers. Chem. Rev. 121, 4805–4902 (2021).
Christie, G. H. & Kenner, J. LXXI.—The molecular configurations of polynuclear aromatic compounds. Part I. The resolution of γ-6:6′-dinitro- and 4:6:4′:6′-tetranitro-diphenic acids into optically active components. J. Chem. Soc. Trans. 121, 614–620 (1922).
Bringmann, G. et al. Atroposelective synthesis of axially chiral biaryl compounds. Angew. Chem. Int. Ed. 44, 5384–5427 (2005).
Smyth, J. E., Butler, N. M. & Keller, P. A. A twist of nature – the significance of atropisomers in biological systems. Nat. Prod. Rep. 32, 1562–1583 (2015).
Clayden, J., Moran, W. J., Edwards, P. J. & LaPlante, S. R. The challenge of atropisomerism in drug discovery. Angew. Chem. Int. Ed. 48, 6398–6401 (2009).
Glunz, P. W. Recent encounters with atropisomerism in drug discovery. Bioorg. Med. Chem. Lett. 28, 53–60 (2018).
Zhang, Y.-B. et al. Single-crystal structure of a covalent organic framework. J. Am. Chem. Soc. 135, 16336–16339 (2013).
Geng, K. et al. Covalent organic frameworks: design, synthesis, and functions. Chem. Rev. 16, 8814–8933 (2020).
Aitipamula, S. et al. Polymorphs, salts, and cocrystals: what’s in a name? Cryst. Growth Des. 12, 2147–2152 (2012).
Piaggi, P. M. & Parrinello, M. Predicting polymorphism in molecular crystals using orientational entropy. Proc. Natl Acad. Sci. USA 115, 10251–10256 (2018).
Widmer, R. N. et al. Rich polymorphism of a metal–organic framework in pressure–temperature space. J. Am. Chem. Soc. 141, 9330–9337 (2019).
Li, Y. et al. Polymorphism of 2D imine covalent organic frameworks. Angew. Chem. Int. Ed. 60, 5363–5369 (2021).
Jones, J. T. A. et al. On–off porosity switching in a molecular organic solid. Angew. Chem. Int. Ed. 50, 749–753 (2011).
Serre, C. et al. Very large breathing effect in the first nanoporous chromium(III)-based solids: MIL-53 or CrIII(OH)·{O2C−C6H4−CO2}·{HO2C−C6H4−CO2H}x·H2Oy. J. Am. Chem. Soc. 124, 13519–13526 (2002).
Wu, C.-D., Hu, A., Zhang, L. & Lin, W. A homochiral porous metal−organic framework for highly enantioselective heterogeneous asymmetric catalysis. J. Am. Chem. Soc. 127, 8940–8941 (2005).
Gong, W., Chen, Z., Dong, J., Liu, Y. & Cui, Y. Chiral metal–organic frameworks. Chem. Rev. 122, 9078–9144 (2022).
Shekunov, B. Y., Aulton, M. E., Adama-Acquah, R. W. & Grant, D. J. W. Effect of temperature on crystal growth and crystal properties of paracetamol. J. Chem. Soc. Faraday Trans. 92, 439–444 (1996).
Judge, R. A., Jacobs, R. S., Frazier, T., Snell, E. H. & Pusey, M. L. The effect of temperature and solution pH on the nucleation of tetragonal lysozyme crystals. Biophys. J. 77, 1585–1593 (1999).
Sun, Y.-X. & Sun, W.-Y. Influence of temperature on metal-organic frameworks. Chin. Chem. Lett. 25, 823–828 (2014).
Krause, S. et al. A pressure-amplifying framework material with negative gas adsorption transitions. Nature 532, 348–352 (2016).
Krause, S. et al. Towards general network architecture design criteria for negative gas adsorption transitions in ultraporous frameworks. Nat. Commun. 10, 3632 (2019).
Garai, B. et al. Reversible switching between positive and negative thermal expansion in a metal–organic framework DUT-49. J. Mater. Chem. A 8, 20420–20428 (2020).
Li, D. & Kaneko, K. Hydrogen bond-regulated microporous nature of copper complex-assembled microcrystals. Chem. Phys. Lett. 335, 50–56 (2001).
Eguchi, R., Uchida, S. & Mizuno, N. Inverse and high CO2/C2H2 sorption selectivity in flexible organic–inorganic ionic crystals. Angew. Chem. Int. Ed. 51, 1635–1639 (2012).
Li, J.-R., Kuppler, R. J. & Zhou, H.-C. Selective gas adsorption and separation in metal–organic frameworks. Chem. Soc. Rev. 38, 1477–1504 (2009).
Cockayne, E. Thermodynamics of the flexible metal–organic framework material MIL-53(Cr) from first-principles. J. Phys. Chem. C 121, 4312–4317 (2017).
Pallach, R. et al. Frustrated flexibility in metal-organic frameworks. Nat. Commun. 12, 4097 (2021).
Sen, S. et al. Cooperative bond scission in a soft porous crystal enables discriminatory gate opening for ethylene over ethane. J. Am. Chem. Soc. 139, 18313–18321 (2017).
Demuynck, R. et al. Efficient construction of free energy profiles of breathing metal–organic frameworks using advanced molecular dynamics simulations. J. Chem. Theory Comput. 13, 5861–5873 (2017).
Sato, H. et al. Self-accelerating CO sorption in a soft nanoporous crystal. Science 343, 167–170 (2014).
Uribe-Romo, F. J. et al. A crystalline imine-linked 3-D porous covalent organic framework. J. Am. Chem. Soc. 131, 4570–4571 (2009).
Ma, Y.-X. et al. A dynamic three-dimensional covalent organic framework. J. Am. Chem. Soc. 139, 4995–4998 (2017).
Poloni, R., Lee, K., Berger, R. F., Smit, B. & Neaton, J. B. Understanding trends in CO2 adsorption in metal–organic frameworks with open-metal sites. J. Phys. Chem. Lett. 5, 861–865 (2014).
Ding, Q. et al. Exploiting equilibrium-kinetic synergetic effect for separation of ethylene and ethane in a microporous metal-organic framework. Sci. Adv. 6, eaaz4322 (2020).
Akkermans, R. L. C., Spenley, N. A. & Robertson, S. H. Monte Carlo methods in Materials Studio. Mol. Simul. 39, 1153–1164 (2013).
Cichocka, M. O., Ångström, J., Wang, B., Zou, X. & Smeets, S. High-throughput continuous rotation electron diffraction data acquisition via software automation. J. Appl. Crystallogr. 51, 1652–1661 (2018).
Kabsch, W. XDS. Acta Crystallogr. D Biol. Crystallogr. 66, 125–132 (2010).
Sheldrick, G. M. A short history of SHELX. Acta Crystallogr. A 64, 112–122 (2008).
Acknowledgements
This work was supported by ExxonMobil through the Singapore Energy Center (EM11161.TO4, EM11161.TO17), the Ministry of Education—Singapore (MOE2019-T2-1-093, MOE-T2EP10122-0002), the Energy Market Authority of Singapore (EMA-EP009-SEGC-020), the Agency for Science, Technology and Research (U2102d2004, U2102d2012), the National Research Foundation Singapore (NRF-CRP26-2021RS-0002), Japan Society for the Promotion of Science KAKENHI (JP19H02734, JP20H02575, JP20K20564), the Swedish Research Council (VR, 2016-04625; 2017-04321) and the Swedish Research Council Formas (2020-00831). We thank the staff from the test centre of the Department of Chemical and Biomolecular Engineering, National University of Singapore, for the assistance with scanning electron microscopy and Fourier-transform infrared spectroscopy measurements.
Author information
Authors and Affiliations
Contributions
D.Z. formulated and supervised the project. C.K. performed the synthesis of COF-320-A crystals and analyses of its growth kinetics and gas sorption measurements. Z.Z. and C.K. performed simulations on the COF–gas interactions. S.K., K.N. and R.M. performed in situ PXRD measurements. Z.H. and X.Z. performed the structure resolving of COF-320-A and COF-320-A2 single crystals. C.K. and D.Z. wrote the manuscript. A.K.U., D.C.C., L.S.B., Y.W., Z.Z., S.K., K.N., X.Z., Z.H., and R.K. contributed to the data analysis, discussion and manuscript revision.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Materials thanks François-Xavier Coudert and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–18 and Tables 1–7.
Supplementary Data 1
Crystal structure of COF-320-A.
Supplementary Data 2
Crystal structure of COF-320-A2.
Supplementary Data 3
Simulated and refined atomistic coordinates of COF-320-A2 during C2H4 adsorption.
Supplementary Data 4
Simulated and refined atomistic coordinates of COF-320-A2E1 during C2H4 adsorption.
Supplementary Data 5
Simulated and refined atomistic coordinates of COF-320-AE1 during CO2 adsorption.
Supplementary Data 6
Simulated and refined atomistic coordinates of COF-320-AE2 during CO2 adsorption.
Supplementary Data 7
Simulated and refined atomistic coordinates of COF-320-AE3 during CO2 adsorption.
Supplementary Data 8
Simulated and refined atomistic coordinates of COF-320-A2E2 during C2H2 adsorption.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kang, C., Zhang, Z., Kusaka, S. et al. Covalent organic framework atropisomers with multiple gas-triggered structural flexibilities. Nat. Mater. 22, 636–643 (2023). https://doi.org/10.1038/s41563-023-01523-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41563-023-01523-2
This article is cited by
-
Dynamic two-dimensional covalent organic frameworks
Nature Chemistry (2024)
-
Composite Nanoarchitectonics Towards Method for Everything in Materials Science
Journal of Inorganic and Organometallic Polymers and Materials (2024)
-
Bulk evidence of anisotropic s-wave pairing with no sign change in the kagome superconductor CsV3Sb5
Nature Communications (2023)
-
Quantum states and intertwining phases in kagome materials
Nature Reviews Physics (2023)
-
Incommensurate charge-stripe correlations in the kagome superconductor CsV3Sb5−xSnx
npj Quantum Materials (2023)