Abstract
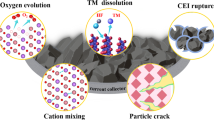
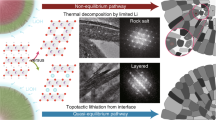
High-Ni-content layered materials are promising cathodes for next-generation lithium-ion batteries. However, investigating the atomic configurations of the delithiation-induced complex phase boundaries and their transitions remains challenging. Here, by using deep-learning-aided super-resolution electron microscopy, we resolve the intralayer transition motifs at complex phase boundaries in high-Ni cathodes. We reveal that an O3 → O1 transformation driven by delithiation leads to the formation of two types of O1–O3 interface, the continuous- and abrupt-transition interfaces. The interfacial misfit is accommodated by a continuous shear-transition zone and an abrupt structural unit, respectively. Atomic-scale simulations show that uneven in-plane Li+ distribution contributes to the formation of both types of interface, and the abrupt transition is energetically more favourable in a delithiated state where O1 is dominant, or when there is an uneven in-plane Li+ distribution in a delithiated O3 lattice. Moreover, a twin-like motif that introduces structural units analogous to the abrupt-type O1–O3 interface is also uncovered. The structural transition motifs resolved in this study provide further understanding of shear-induced phase transformations and phase boundaries in high-Ni layered cathodes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author on reasonable request.
Code availability
The code used to perform super-resolution processing can be accessed at temimagenet.com.
References
Li, W., Erickson, E. M. & Manthiram, A. High-nickel layered oxide cathodes for lithium-based automotive batteries. Nat. Energy 5, 26–34 (2020).
Voronina, N., Sun, Y.-K. & Myung, S.-T. Co-free layered cathode materials for high energy density lithium-ion batteries. ACS Energy Lett. 5, 1814–1824 (2020).
Kim, U. H. et al. Microstructure‐controlled Ni‐rich cathode material by microscale compositional partition for next‐generation electric vehicles. Adv. Energy Mater. 9, 1803902 (2019).
Bi, Y. et al. Reversible planar gliding and microcracking in a single-crystalline Ni-rich cathode. Science 370, 1313–1317 (2020).
Trevisanello, E., Ruess, R., Conforto, G., Richter, F. H. & Janek, J. Polycrystalline and single crystalline NCM cathode materials—quantifying particle cracking, active surface area, and lithium diffusion. Adv. Energy Mater. 11, 2003400 (2021).
Lin, R. et al. Hierarchical nickel valence gradient stabilizes high-nickel content layered cathode materials. Nat. Commun. 12, 2350 (2021).
Zhang, R. et al. Compositionally complex doping for zero-strain zero-cobalt layered cathodes. Nature 610, 67–73 (2022).
Yan, P. et al. Coupling of electrochemically triggered thermal and mechanical effects to aggravate failure in a layered cathode. Nat. Commun. 9, 2437 (2018).
Yan, P. et al. Intragranular cracking as a critical barrier for high-voltage usage of layer-structured cathode for lithium-ion batteries. Nat. Commun. 8, 14101 (2017).
Mu, L. et al. Oxygen release induced chemomechanical breakdown of layered cathode materials. Nano Lett. 18, 3241–3249 (2018).
Gabrisch, H., Yazami, R. & Fultz, B. The character of dislocations in LiCoO2. Electrochem. Solid-State Lett. 5, A111–A114 (2002).
Croguennec, L., Pouillerie, C. & Delmas, C. NiO2 obtained by electrochemical lithium deintercalation from lithium nickelate: structural modifications. J. Electrochem. Soc. 147, 1314 (2000).
Lee, S. et al. Oxygen vacancy diffusion and condensation in lithium-ion battery cathode materials. Angew. Chem. Int. Ed. 58, 10478–10485 (2019).
Hu, E. et al. Evolution of redox couples in Li- and Mn-rich cathode materials and mitigation of voltage fade by reducing oxygen release. Nat. Energy 3, 690–698 (2018).
Hu, E. et al. Oxygen-release-related thermal stability and decomposition pathways of LixNi0.5Mn1.5O4 cathode materials. Chem. Mater. 26, 1108–1118 (2013).
Bak, S. M. et al. Structural changes and thermal stability of charged LiNixMnyCozO2 cathode materials studied by combined in situ time-resolved XRD and mass spectroscopy. ACS Appl. Mater. Interfaces 6, 22594–22601 (2014).
Lin, F. et al. Surface reconstruction and chemical evolution of stoichiometric layered cathode materials for lithium-ion batteries. Nat. Commun. 5, 3529 (2014).
Xu, C. et al. Bulk fatigue induced by surface reconstruction in layered Ni-rich cathodes for Li-ion batteries. Nat. Mater. 20, 84–92 (2020).
Wang, C. et al. Resolving atomic-scale phase transformation and oxygen loss mechanism in ultrahigh-nickel layered cathodes for cobalt-free lithium-ion batteries. Matter 4, 2013–2026 (2021).
Xu, C., Reeves, P. J., Jacquet, Q. & Grey, C. P. Phase behavior during electrochemical cycling of Ni‐rich cathode materials for Li‐ion batteries. Adv. Energy Mater. 11, 2003404 (2020).
Radin, M. D. et al. Narrowing the gap between theoretical and practical capacities in Li-ion layered oxide cathode materials. Adv. Energy Mater. 7, 1602888 (2017).
Chen, Z., Lu, Z. & Dahn, J. R. Staging phase transitions in LixCoO2. J. Electrochem. Soc. 149, A1604 (2002).
Wang, C., Zhang, R., Kisslinger, K. & Xin, H. L. Atomic-scale observation of O1 faulted phase-induced deactivation of LiNiO2 at high voltage. Nano Lett. 21, 3657–3663 (2021).
Bianchini, M., Roca-Ayats, M., Hartmann, P., Brezesinski, T. & Janek, J. There and back again—the journey of LiNiO2 as a cathode active material. Angew. Chem. Int. Ed. 58, 10434–10458 (2019).
Yan, P. et al. Injection of oxygen vacancies in the bulk lattice of layered cathodes. Nat. Nanotechnol. 14, 602–608 (2019).
Yoon, C. S., Jun, D.-W., Myung, S.-T. & Sun, Y.-K. Structural stability of LiNiO2 cycled above 4.2 V. ACS Energy Lett. 2, 1150–1155 (2017).
Wang, C. et al. Chemomechanically stable ultrahigh-Ni single-crystalline cathodes with improved oxygen retention and delayed phase degradations. Nano Lett. 21, 9797–9804 (2021).
Lin, R., Zhang, R., Wang, C., Yang, X. Q. & Xin, H. L. TEMImageNet training library and AtomSegNet deep-learning models for high-precision atom segmentation, localization, denoising, and deblurring of atomic-resolution images. Sci. Rep. 11, 5386 (2021).
Du, K., Rau, Y., Jin-Phillipp, N. Y. & Phillipp, F. Lattice distortion analysis directly from high resolution transmission electron microscopy images—the LADIA program package. J. Mater. Sci. Technol. 18, 135–138 (2002).
Wang, C. et al. Size-dependent grain-boundary structure with improved conductive and mechanical stabilities in sub-10-nm gold crystals. Phys. Rev. Lett. 120, 186102 (2018).
Wang, S. J. et al. Deformation-induced structural transition in body-centred cubic molybdenum. Nat. Commun. 5, 3433 (2014).
Tu, Q., Barroso-Luque, L., Shi, T. & Ceder, G. Electrodeposition and mechanical stability at lithium-solid electrolyte interface during plating in solid-state batteries. Cell Rep. Phys. Sci. 1, 100106 (2020).
Wang, C. et al. Three-dimensional atomic structure of grain boundaries resolved by atomic-resolution electron tomography. Matter 3, 1999–2011 (2020).
Fu, X. et al. A high-performance carbonate-free lithium|garnet interface enabled by a trace amount of sodium. Adv. Mater. 32, e2000575 (2020).
Xue, S. et al. The formation mechanisms of growth twins in polycrystalline Al with high stacking fault energy. Acta Mater. 101, 62–70 (2015).
Song, M. et al. Oriented attachment induces fivefold twins by forming and decomposing high-energy grain boundaries. Science 367, 40–45 (2020).
Wang, J. et al. In situ atomic-scale observation of twinning-dominated deformation in nanoscale body-centred cubic tungsten. Nat. Mater. 14, 594–600 (2015).
Li, W., Song, B. & Manthiram, A. High-voltage positive electrode materials for lithium-ion batteries. Chem. Soc. Rev. 46, 3006–3059 (2017).
Sun, C. et al. High-voltage cycling induced thermal vulnerability in LiCoO2 cathode: cation loss and oxygen release driven by oxygen vacancy migration. ACS Nano 14, 6181–6190 (2020).
Lai, J. et al. Structural elucidation of the degradation mechanism of nickel-rich layered cathodes during high-voltage cycling. Chem. Commun. 56, 4886–4889 (2020).
Kresse, G. & Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996).
Blochl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Dudarev, S. L., Botton, G. A., Savrasov, S. Y., Humphreys, C. J. & Sutton, A. P. Electron-energy-loss spectra and the structural stability of nickel oxide: An LSDA+U study. Phys. Rev. B 57, 1505–1509 (1998).
Jain, A. et al. A high-throughput infrastructure for density functional theory calculations. Comput. Mater. Sci. 50, 2295–2310 (2011).
Acknowledgements
This work is supported by the Materials Science and Engineering Divisions, Office of Basic Energy Sciences of the US Department of Energy, under award no. DE-SC0021204. R.Z.’s work done for this study was funded by H.L.X.’s startup funding. This research used resources of the Center for Functional Nanomaterials, which is a US DOE Office of Science Facility, and the Scientific Data and Computing Center, a component of the Computational Science Initiative, at Brookhaven National Laboratory under contract no. DE-SC0012704. We acknowledge the use of facilities and instrumentation at the University of California, Irvine Materials Research Institute, which is supported in part by the National Science Foundation through the University of California, Irvine Materials Research Science and Engineering Center (no. DMR-2011967). We would like to acknowledge the generous support from Professor Tim Rupert. We thank Y. Cheng and J. Cheng for illuminating discussions.
Author information
Authors and Affiliations
Contributions
H.L.X. directed the project. C.W. and H.L.X. conceived the idea. C.W. performed the transmission electron microscopy experiments and data analysis. X.W. performed the DFT simulations and data interpretation. R.Z. performed the materials synthesis and electrochemical tests. C.W. performed the four-dimensional STEM strain mapping and data analysis. C.W. and T.L. performed the scanning nanodiffraction experiments for orientation mapping. K.K. prepared the focused ion beam transmission electron microscopy samples. C.W., X.W. and H.L.X. wrote the paper with help from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Materials thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–14 and Discussion.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, C., Wang, X., Zhang, R. et al. Resolving complex intralayer transition motifs in high-Ni-content layered cathode materials for lithium-ion batteries. Nat. Mater. 22, 235–241 (2023). https://doi.org/10.1038/s41563-022-01461-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41563-022-01461-5
This article is cited by
-
Long-life lithium-ion batteries realized by low-Ni, Co-free cathode chemistry
Nature Energy (2023)
-
Defective oxygen inert phase stabilized high-voltage nickel-rich cathode for high-energy lithium-ion batteries
Nature Communications (2023)