Abstract
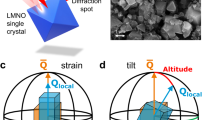
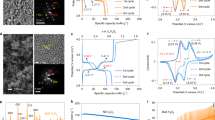
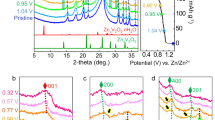
Electrochemical phase transformation in ion-insertion crystalline electrodes is accompanied by compositional and structural changes, including the microstructural development of oriented phase domains. Previous studies have identified prevailingly transformation heterogeneities associated with diffusion- or reaction-limited mechanisms. In comparison, transformation-induced domains and their microstructure resulting from the loss of symmetry elements remain unexplored, despite their general importance in alloys and ceramics. Here, we map the formation of oriented phase domains and the development of strain gradient quantitatively during the electrochemical ion-insertion process. A collocated four-dimensional scanning transmission electron microscopy and electron energy loss spectroscopy approach, coupled with data mining, enables the study. Results show that in our model system of cubic spinel MnO2 nanoparticles their phase transformation upon Mg2+ insertion leads to the formation of domains of similar chemical identity but different orientations at nanometre length scale, following the nucleation, growth and coalescence process. Electrolytes have a substantial impact on the transformation microstructure (‘island’ versus ‘archipelago’). Further, large strain gradients build up from the development of phase domains across their boundaries with high impact on the chemical diffusion coefficient by a factor of ten or more. Our findings thus provide critical insights into the microstructure formation mechanism and its impact on the ion-insertion process, suggesting new rules of transformation structure control for energy storage materials.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The 4D-STEM and EELS data associated with this paper can be found at https://doi.org/10.13012/B2IDB-4717991_V1. Additional data are available on request from the corresponding authors.
Code availability
The codes used for the 4D-STEM strain mapping, oriented phase domain analysis, EELS analysis, radial distribution function and pair correlation function calculations can be accessed at https://github.com/chenlabUIUC/OrientedPhaseDomain. Additional codes are available on request from the corresponding authors.
References
Stupp, S. I. & Braun, P. V. Molecular manipulation of microstructures: biomaterials, ceramics, and semiconductors. Science 277, 1242–1248 (1997).
Dahmen, U. in Encyclopedia of Physical Science and Technology (ed. Meyers, R. A.) 821–853 (Elsevier, 2003).
Xu, Z. et al. Charge distribution guided by grain crystallographic orientations in polycrystalline battery materials. Nat. Commun. 11, 83 (2020).
Gerstein, G., L’vov, V. A., Zak, A., Dudzinski, W. & Maier, H. J. Direct observation of nano-dimensional internal structure of ferromagnetic domains in the ferromagnetic shape memory alloy Co–Ni–Ga. J. Magn. Magn. Mater. 466, 125–129 (2018).
Katayama, K. et al. Orientation control and domain structure analysis of {100}-oriented epitaxial ferroelectric orthorhombic HfO2-based thin films. J. Appl. Phys. 119, 134101 (2016).
Sun, L. G., He, X. Q. & Lu, J. Nanotwinned and hierarchical nanotwinned metals: a review of experimental, computational and theoretical efforts. npj Comput. Mater. 4, 6 (2018).
Thackeray, M. M. & Amine, K. LiMn2O4 spinel and substituted cathodes. Nat. Energy 6, 566–566 (2021).
Chiang, Y. M., Wang, H. F. & Jang, Y. I. Electrochemically induced cation disorder and phase transformations in lithium intercalation oxides. Chem. Mater. 13, 53–63 (2001).
Kim, C. et al. Direct observation of reversible magnesium ion intercalation into a spinel oxide host. Adv. Mater. 27, 3377–3384 (2015).
Merryweather, A. J., Schnedermann, C., Jacquet, Q., Grey, C. P. & Rao, A. Operando optical tracking of single-particle ion dynamics in batteries. Nature 594, 522–528 (2021).
Peres, J. P., Weill, F. & Delmas, C. Lithium/vacancy ordering in the monoclinic LixNiO2 (0.50 ≤ x ≤ 0.75) solid solution. Solid State Ion. 116, 19–27 (1999).
Shao-Horn, Y., Levasseur, S., Weill, F. & Delmas, C. Probing lithium and vacancy ordering in O3 layered LixCoO2 (x ≈ 0.5): an electron diffraction study. J. Electrochem. Soc. 150, A366 (2003).
Berthelot, R., Carlier, D. & Delmas, C. Electrochemical investigation of the P2–NaxCoO2 phase diagram. Nat. Mater. 10, 74–80 (2011).
Ben Yahia, H., Shikano, M. & Kobayashi, H. Phase transition mechanisms in LixCoO2 (0.25 ≤ x ≤ 1) based on group–subgroup transformations. Chem. Mater. 25, 3687–3701 (2013).
Gu, M. et al. Formation of the spinel phase in the layered composite cathode used in Li-ion batteries. ACS Nano 7, 760–767 (2013).
Shukla, A. K. et al. Unravelling structural ambiguities in lithium- and manganese-rich transition metal oxides. Nat. Commun. 6, 8711 (2015).
Jarvis, K. A. et al. Formation and effect of orientation domains in layered oxide cathodes of lithium-ion batteries. Acta Mater. 108, 264–270 (2016).
Shukla, A. K. et al. Effect of composition on the structure of lithium- and manganese-rich transition metal oxides. Energy Environ. Sci. 11, 830–840 (2018).
Shao-Horn, Y. et al. Lithium and vacancy ordering in T#2−LixCoO2 derived from O2-type LiCoO2. Chem. Mater. 15, 2977–2983 (2003).
Kuhne, M. et al. Reversible superdense ordering of lithium between two graphene sheets. Nature 564, 234–239 (2018).
Sun, H. B. et al. Tailoring disordered/ordered phases to revisit the degradation mechanism of high-voltage LiNi0.5Mn1.5O4 spinel cathode materials. Adv. Funct. Mater. 32, 2112279 (2022).
Lim, J. et al. Origin and hysteresis of lithium compositional spatiodynamics within battery primary particles. Science 353, 566–571 (2016).
Deng, H. D. et al. Correlative image learning of chemo-mechanics in phase-transforming solids. Nat. Mater. 21, 547–554 (2022).
Ulvestad, A. et al. Topological defect dynamics in operando battery nanoparticles. Science 348, 1344–1347 (2015).
Han, S. Y. et al. Stress evolution during cycling of alloy-anode solid-state batteries. Joule 5, 2450–2465 (2021).
Chen, W. et al. Effects of particle size on Mg2+ ion intercalation into λ-MnO2 cathode materials. Nano Lett. 19, 4712–4720 (2019).
Canepa, P. et al. Odyssey of multivalent cathode materials: open questions and future challenges. Chem. Rev. 117, 4287–4341 (2017).
Lin, F. et al. Synchrotron X-ray analytical techniques for studying materials electrochemistry in rechargeable batteries. Chem. Rev. 117, 13123–13186 (2017).
Lewis, J. A. et al. Linking void and interphase evolution to electrochemistry in solid-state batteries using operando X-ray tomography. Nat. Mater. 20, 503–510 (2021).
Yin, Z. W. et al. Advanced electron energy loss spectroscopy for battery studies. Adv. Funct. Mater. 32, 2107190 (2021).
Ophus, C. Four-dimensional scanning transmission electron microscopy (4D-STEM): from scanning nanodiffraction to ptychography and beyond. Microsc. Microanal. 25, 563–582 (2019).
Liu, X. et al. Local electronic structure variation resulting in Li ‘filament’ formation within solid electrolytes. Nat. Mater. 20, 1485–1490 (2021).
Park, J. et al. Fictitious phase separation in Li layered oxides driven by electro-autocatalysis. Nat. Mater. 20, 991–999 (2021).
Cogswell, D. A. & Bazant, M. Z. Coherency strain and the kinetics of phase separation in LiFePO4 nanoparticles. ACS Nano 6, 2215–2225 (2012).
Gibot, P. et al. Room-temperature single-phase Li insertion/extraction in nanoscale LixFePO4. Nat. Mater. 7, 741–747 (2008).
Liang, Y., Dong, H., Aurbach, D. & Yao, Y. Current status and future directions of multivalent metal-ion batteries. Nat. Energy 5, 646–656 (2020).
Hou, S. et al. Solvation sheath reorganization enables divalent metal batteries with fast interfacial charge transfer kinetics. Science 374, 172–178 (2021).
Lu, J., Chen, Z., Pan, F., Cui, Y. & Amine, K. High-performance anode materials for rechargeable lithium-ion batteries. Electrochem. Energy Rev. 1, 35–53 (2018).
Sood, A. et al. Electrochemical ion insertion from the atomic to the device scale. Nat. Rev. Mater. 6, 847–867 (2021).
Padgett, E. et al. The exit-wave power-cepstrum transform for scanning nanobeam electron diffraction: robust strain mapping at subnanometer resolution and subpicometer precision. Ultramicroscopy 214, 112994 (2020).
Malik, R., Zhou, F. & Ceder, G. Kinetics of non-equilibrium lithium incorporation in LiFePO4. Nat. Mater. 10, 587–590 (2011).
Laffont, L. & Gibot, P. High resolution electron energy loss spectroscopy of manganese oxides: application to Mn3O4 nanoparticles. Mater. Charact. 61, 1268–1273 (2010).
Li, J. et al. Size-dependent kinetics during non-equilibrium lithiation of nano-sized zinc ferrite. Nat. Commun. 10, 93 (2019).
Rouviere, J. L. & Sarigiannidou, E. Theoretical discussions on the geometrical phase analysis. Ultramicroscopy 106, 1–17 (2005).
Shao, Y. T. et al. Cepstral scanning transmission electron microscopy imaging of severe lattice distortions. Ultramicroscopy 231, 113252 (2021).
Ulvestad, A. et al. Single particle nanomechanics in operando batteries via lensless strain mapping. Nano Lett. 14, 5123–5127 (2014).
Jin, B., Sushko, M. L., Liu, Z., Jin, C. & Tang, R. In situ liquid cell TEM reveals bridge-induced contact and fusion of Au nanocrystals in aqueous solution. Nano Lett. 18, 6551–6556 (2018).
Sun, W. et al. Zn/MnO2 battery chemistry with H+ and Zn2+ coinsertion. J. Am. Chem. Soc. 139, 9775–9778 (2017).
Verma, A. et al. Galvanostatic intermittent titration and performance based analysis of LiNi0.5Co0.2Mn0.3O2 cathode. J. Electrochem. Soc. 164, A3380–A3392 (2017).
Spence, J. C. H. & Zuo, J.-M. Electron Microdiffraction (Springer, 1992).
Acknowledgements
This work was primarily (sample preparation, majority of the 4D-STEM, EELS, XRD characterizations, electrochemical testing and data analysis) supported by the US Department of Energy, Office of Basic Energy Sciences, Division of Materials Sciences and Engineering, under award DE-SC0022035 (W.C., Z.T. and Q.C.). Some of the 4D-STEM experiments and the atomic-scale imaging were supported by Intel Corporation through an SRC project, award 54071821 (R.Y. and J.-M.Z.) and the Energy & Biosciences Institute through the EBI-Shell programme (W.C., S.P., C.Z., H.Y., J.-M.Z. and Q.C.). The DFT calculation and GITT and EIS measurements were supported in part by the US Army Construction Engineering Research Laboratory, Temperature Insensitive High-Density Lithium-Ion Batteries, award W9132T-21-2-0008 (A.X.B.Y., H.J., E.E., A.P. and P.V.B.). The preparation of dry solvents in the glovebox was supported in part by the Joint Center for Energy Storage Research (JCESR), an Energy Innovation Hub funded by the US Department of Energy, Office of Science, Basic Energy Sciences (K.T. and A.A.G.). The analysis of XRD data by Rietveld refinement was supported by a DIGI-MAT fellowship from the NSF DGE programme, award 1922758 (Z.W.R. and D.P.S.) using instrumentation partially supported by NSF through the University of Illinois Materials Research Science and Engineering Center DMR-172063. We thank Z. Ou and J. W. Smith at the University of Illinois for discussions on radial distribution function. We thank C. Qian and L. Yao at the University of Illinois for assistance with MATLAB coding in the analysis of the radial distribution functions and strain gradients. We thank N. Admal at the University of Illinois for discussions on domain morphology and phase separation. We thank Y. Yang at Columbia University for discussions on electrochemical tests.
Author information
Authors and Affiliations
Contributions
W.C., J.-M.Z. and Q.C. planned the research and led the project. W.C. performed material processing and conducted electrochemical measurements. X.Z., R.Y., W.C., S.P. and K.Y. conducted the 4D-STEM and STEM imaging. S.P., W.C., X.Z. and K.Y. conducted EELS mapping. W.C., X.Z., R.Y., J.-M.Z. and Q.C. analysed and interpreted the electron microscopy data. W.C., with assistance from H.A. and A.P., analysed and interpreted the electrochemical results. Z.W.R. and D.P.S. performed the analysis of XRD data by Rietveld refinement. A.X.B.Y., H.J. and E.E. performed the DFT calculations. Z.T., C.Z., K.T. and H.Y. assisted in sample preparation. A.P., H.A., P.V.B. and A.A.G. assisted with GITT and EIS measurements. H.A. assisted with preparation of schematics. W.C., J.-M. Z. and Q.C. wrote the manuscript. R.M.S., J.-M.Z. and Q.C. supervised the research. All authors contributed to the discussion of the results.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Materials thanks Matthew McDowell and Colin Ophus for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Notes 1‒7, Tables 1‒3, Figs. 1‒30 and refs.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, W., Zhan, X., Yuan, R. et al. Formation and impact of nanoscopic oriented phase domains in electrochemical crystalline electrodes. Nat. Mater. 22, 92–99 (2023). https://doi.org/10.1038/s41563-022-01381-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41563-022-01381-4
This article is cited by
-
Visualizing the multi-level assembly structures of conjugated molecular systems with chain-length dependent behavior
Nature Communications (2023)
-
Control of metal oxides’ electronic conductivity through visual intercalation chemical reactions
Nature Communications (2023)
-
Asynchronous domain dynamics and equilibration in layered oxide battery cathode
Nature Communications (2023)