Abstract
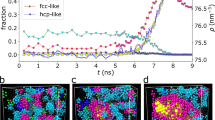
It is believed that the slow liquid diffusion and geometric frustration brought by a rapid, deep quench inhibit fast crystallization and promote vitrification. Here we report fast crystal growth in charged colloidal systems under deep supercooling, where liquid diffusion is extremely low. By combining experiments and simulations, we show that this process occurs via wall-induced barrierless ordering consisting of two coupled steps: the step-like advancement of the rough interface that disintegrates frustration, followed by defect repairing inside the newly formed solid phase. The former is a diffusionless collective process, whereas the latter controls crystal quality. We further show that the intrinsic mechanical instability of a disordered glassy state subject to the crystal growth front allows for domino-like fast crystal growth even at ultra-low temperatures. These findings contribute to a deeper understanding of fast crystal growth and may be useful for applications related to vitrification prevention and crystal-quality control.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Source data are provided with this paper. All other data that support the plots within this paper and other findings of this study are available from the corresponding authors upon reasonable request.
Code availability
The computer codes used in this paper are available from the corresponding authors upon reasonable request.
References
Broughton, J., Gilmer, G. & Jackson, K. Crystallization rates of a Lennard-Jones liquid. Phys. Rev. Lett. 49, 1496–1500 (1982).
Jackson, K. A., Gilmer, G. H., Temkin, D. E., Weinberg, J. D. & Beatty, K. Non-equilibrium phase transformations. J. Cryst. Growth 128, 127–138 (1993).
Hikima, T., Hanaya, M. & Oguni, M. Microscopic observation of a peculiar crystallization in the glass transition region and β-process as potentially controlling the growth rate in triphenylethylene. J. Mol. Struct. 479, 245–250 (1999).
Tanaka, H. Possible resolution of the Kauzmann paradox in supercooled liquids. Phys. Rev. E 68, 011505 (2003).
Konishi, T. & Tanaka, H. Possible origin of enhanced crystal growth in a glass. Phys. Rev. B 76, 220201 (2007).
Ediger, M., Harrowell, P. & Yu, L. Crystal growth kinetics exhibit a fragility-dependent decoupling from viscosity. J. Chem. Phys. 128, 034709 (2008).
Zaccarelli, E. et al. Crystallization of hard-sphere glasses. Phys. Rev. Lett. 103, 135704 (2009).
Orava, J. & Greer, A. Fast and slow crystal growth kinetics in glass-forming melts. J. Chem. Phys. 140, 214504 (2014).
Gránásy, L. et al. Growth of ‘dizzy dendrites’ in a random field of foreign particles. Nat. Mater. 2, 92–96 (2003).
Gránásy, L., Pusztai, T., Börzsönyi, T., Warren, J. A. & Douglas, J. F. A general mechanism of polycrystalline growth. Nat. Mater. 3, 645–650 (2004).
Jackson, K. A. The interface kinetics of crystal growth processes. Interface Sci. 10, 159–169 (2002).
Sear, R. P. Nucleation in the presence of slow microscopic dynamics. J. Chem. Phys. 128, 214513 (2008).
Tegze, G. et al. Diffusion-controlled anisotropic growth of stable and metastable crystal polymorphs in the phase-field crystal model. Phys. Rev. Lett. 103, 035702 (2009).
Wilson, H. W. On the velocity of solidification and viscosity of super-cooled liquids. Philos. Mag. 50, 238–250 (1900).
Born, M. & Green, H. S. A General Kinetic Theory of Liquids (Cambridge Univ. Press, 1949).
Coriell, S. & Turnbull, D. Relative roles of heat transport and interface rearrangement rates in the rapid growth of crystals in undercooled melts. Acta Metall. 30, 2135–2139 (1982).
Kawasaki, T. & Tanaka, H. Formation of a crystal nucleus from liquid. Proc. Natl Acad. Sci. USA 107, 14036–14041 (2010).
Russo, J. & Tanaka, H. The microscopic pathway to crystallization in supercooled liquids. Sci. Rep. 2, 505 (2012).
Tan, P., Xu, N. & Xu, L. Visualizing kinetic pathways of homogeneous nucleation in colloidal crystallization. Nat. Phys. 10, 73–79 (2014).
Kratzer, K. & Arnold, A. Two-stage crystallization of charged colloids under low supersaturation conditions. Soft Matter 11, 2174–2182 (2015).
Watanabe, K., Kawasaki, T. & Tanaka, H. Structural origin of enhanced slow dynamics near a wall in glass-forming systems. Nat. Mater. 10, 512–520 (2011).
Dijkstra, M. & van Roij, R. Entropic wetting in colloidal suspensions. J. Phys. Condens. Matter 17, S3507–S3514 (2005).
Page, A. & Sear, R. Freezing in the bulk controlled by prefreezing at a surface. Phys. Rev. E 80, 031605 (2009).
Shintani, H. & Tanaka, H. Frustration on the way to crystallization in glass. Nat. Phys. 2, 200–206 (2006).
Sun, G., Xu, J. & Harrowell, P. The mechanism of the ultrafast crystal growth of pure metals from their melts. Nat. Mater. 17, 881–886 (2018).
Hwang, H., Weitz, D. A. & Spaepen, F. Direct observation of crystallization and melting with colloids. Proc. Natl Acad. Sci. USA 116, 1180–1184 (2019).
Tanaka, H., Tong, H., Shi, R. & Russo, J. Revealing key structural features hidden in liquids and glasses. Nat. Rev. Phys. 1, 333–348 (2019).
Ashkenazy, Y. & Averback, R. S. Kinetic stages in the crystallization of deeply undercooled body-centered-cubic and face-centered-cubic metals. Acta Mater. 58, 524–530 (2010).
Tóth, G. I., Pusztai, T., Tegze, G., Tóth, G. & Gránásy, L. Amorphous nucleation precursor in highly nonequilibrium fluids. Phys. Rev. Lett. 107, 175702 (2011).
Tanaka, H. Bond orientational order in liquids: towards a unified description of water-like anomalies, liquid-liquid transition, glass transition, and crystallization. Eur. Phys. J. E 35, 113 (2012).
Ashkenazy, Y. & Averback, R. S. Atomic mechanisms controlling crystallization behaviour in metals at deep undercoolings. Europhys. Lett. 79, 26005 (2007).
Tegze, G., Tóth, G. I. & Gránásy, L. Faceting and branching in 2D crystal growth. Phys. Rev. Lett. 106, 195502 (2011).
Aziz, M. J. Model for solute redistribution during rapid solidification. J. Appl. Phys. 53, 1158–1168 (1982).
Dullens, R. P., Aarts, D. G. & Kegel, W. K. Dynamic broadening of the crystal-fluid interface of colloidal hard spheres. Phys. Rev. Lett. 97, 228301 (2006).
Gasser, U., Weeks, E. R., Schofield, A., Pusey, P. & Weitz, D. Real-space imaging of nucleation and growth in colloidal crystallization. Science 292, 258–262 (2001).
Arai, S. & Tanaka, H. Surface-assisted single-crystal formation of charged colloids. Nat. Phys. 13, 503–509 (2017).
Wang, Z., Wang, F., Peng, Y., Zheng, Z. & Han, Y. Imaging the homogeneous nucleation during the melting of superheated colloidal crystals. Science 338, 87–90 (2012).
Alsayed, A. M., Islam, M. F., Zhang, J., Collings, P. J. & Yodh, A. G. Premelting at defects within bulk colloidal crystals. Science 309, 1207–1210 (2005).
Peng, Y., Wang, Z., Alsayed, A. M., Yodh, A. G. & Han, Y. Melting of colloidal crystal films. Phys. Rev. Lett. 104, 205703 (2010).
Li, B. et al. Modes of surface premelting in colloidal crystals composed of attractive particles. Nature 531, 485–488 (2016).
Würth, M., Schwarz, J., Culis, F., Leiderer, P. & Palberg, T. Growth kinetics of body centered cubic colloidal crystals. Phys. Rev. E 52, 6415 (1995).
Palberg, T. Crystallization kinetics of repulsive colloidal spheres. J. Phys. Condens. Matter 11, R323–R360 (1999).
Hynninen, A.-P. & Dijkstra, M. Phase diagrams of hard-core repulsive Yukawa particles. Phys. Rev. E 68, 021407 (2003).
Russo, J. & Tanaka, H. Crystal nucleation as the ordering of multiple order parameters. J. Chem. Phys. 145, 211801 (2016).
Wang, R., Xu, L.-M. & Wang, F. Molecular-scale processes affecting growth rates of ice at moderate supercooling. Front Phys. Beijing 13, 138116 (2018).
Oxtoby, D. W. & Harrowell, P. R. The effect of density change on crystal growth rates from the melt. J. Chem. Phys. 96, 3834–3843 (1992).
Ganapathi, D., Chakrabarti, D., Sood, A. & Ganapathy, R. Structure determines where crystallization occurs in a soft colloidal glass. Nat. Phys. 17 114–120 (2020).
Podmaniczky, F., Tóth, G. I., Tegze, G. & Gránásy, L. Hydrodynamic theory of freezing: nucleation and polycrystalline growth. Phys. Rev. E 95, 052801 (2017).
Delaey, L. Diffusionless Transformations, Phase Transformations in Materials (Wiley-VCH, 2001).
Tang, S., Wang, J., Svendsen, B. & Raabe, D. Competitive bcc and fcc crystal nucleation from non-equilibrium liquids studied by phase-field crystal simulation. Acta Mater. 139, 196–204 (2017).
Tóth, G. I., Tegze, G., Pusztai, T. & Gránásy, L. Heterogeneous crystal nucleation: the effect of lattice mismatch. Phys. Rev. Lett. 108, 025502 (2012).
Shibuta, Y. et al. Heterogeneity in homogeneous nucleation from billion-atom molecular dynamics simulation of solidification of pure metal. Nat. Commun. 8, 10 (2017).
Zhong, R., Kulovits, A., Wiezorek, J. & Leonard, J. Four-zone solidification microstructure formed by laser melting of copper thin films. Appl. Surf. Sci. 256, 105–111 (2009).
Walden, P. Organic solvents and ionization media. III. Interior friction and its relation to conductivity. Z. Phys. Chem. 55, 207–249 (1906).
Royall, C., Leunissen, M. & van Blaaderen, A. A new colloidal model system to study long-range interactions quantitatively in real space. J. Phys. Condens. Matter 15, S3581–S3596 (2003).
Maxwell-Garnett, J. C. Colours in metal glasses and in metallic films. Philos. Trans. R. Soc. A 203, 385–420 (1904).
Plimpton, S. Fast Parallel Algorithms for Short-Range Molecular Dynamics (Sandia National Labs, 1993).
Nosé, S. A unified formulation of the constant temperature molecular dynamics methods. J. Chem. Phys. 81, 511–519 (1984).
Bitzek, E., Koskinen, P., Gähler, F., Moseler, M. & Gumbsch, P. Structural relaxation made simple. Phys. Rev. Lett. 97, 170201 (2006).
Crocker, J. C. & Grier, D. G. Methods of digital video microscopy for colloidal studies. J. Colloid Interface Sci. 179, 298–310 (1996).
Steinhardt, P. J., Nelson, D. R. & Ronchetti, M. Bond-orientational order in liquids and glasses. Phys. Rev. B 28, 784 (1983).
Lechner, W. & Dellago, C. Accurate determination of crystal structures based on averaged local bond order parameters. J. Chem. Phys. 129, 114707 (2008).
Mickel, W., Kapfer, S. C., Schröder-Turk, G. E. & Mecke, K. Shortcomings of the bond orientational order parameters for the analysis of disordered particulate matter. J. Chem. Phys. 138, 044501 (2013).
Acknowledgements
This work is supported by National Natural Science Foundation of China grant no. 11774059, no. 11734014, no. 11935002, no. 11525520, no. 11725521 and no. 12035004; Science and Technology Commission of Shanghai Municipality under grant no. 20JC1414700 and Shanghai Rising Star programme grant no. 16QA1400600; National Key Research and Development Program of China (grant no. 2016YFA0300901); and Hong Kong RGC (GRF 14306518 and GRF 14303415). H. Tanaka acknowledges Grants-in-Aid for Scientific Research (A) (JP18H03675) and Specially Promoted Research (JP25000002 and JP20H05619) from the Japan Society of the Promotion of Science.
Author information
Authors and Affiliations
Contributions
P.T., H. Tanaka and Limei Xu supervised the project. P.T., Q.G. and S.T. performed the experiments, J.A. and H. Tong performed the numerical simulations. All authors contributed to the data analysis. P.T. and H. Tanaka wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review Information Nature Materials thanks László Gránásy and Thomas Palberg for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Layering property of interface particles.
a, Histogram of La(i) for all particles at the initial crystal growth stage. It can efficiently distinguish layered particles from unlayered particles. b, Histogram of ξ(i) for the layered particles in a. The layered particles at interface contains both the solid surface and interface liquid. c, Histogram of La(i) for solid surface particles and interface liquid particles respectively.
Extended Data Fig. 2 Particle counts in the \({L}_{a}-{\overline{q}}_{6}\) parameter space for experiments and simulations.
a,κσ ~ 2.0, T ~ 0.6Tm in experiments. b,κσ ~ 2.0, T ~ 0.6Tm in simulations. c,κσ ~ 3.2, T ~ 0.4Tm in experiments. d,κσ ~ 3.2, T ~ 0.4Tm in simulations. e,κσ ~ 5.0, T ~ 0.5Tm in experiments. f,κσ ~ 5.0, T ~ 0.5Tm in simulations. We can identify the layering of liquid as the region spreading out from the solid part (the upper-left island in each panel) towards the unlayered liquid (the lower-right island in each panel). g, Time evolution of the particle counts in the \({L}_{a}-{\overline{q}}_{6}\) parameter space. From left to right: the initial, early-growth, late-growth, and final stages.
Extended Data Fig. 3 Comparison of the crystallization process with a flat wall and in bulk in simulations at various T (κσ ~ 2.0).
a, The solid fraction versus the time t for samples with a flat wall. We observe a fast wall-assisted crystallization until the growth front meets the small crystal nucleus caused by spinodal nucleation inside the sample (see inset figure). b, The solid fraction versus the time t for bulk samples. The crystallization speed is much lower than in a, which is approximately indicated by the dashed line.
Extended Data Fig. 4 The rough and thick interface profile at various T (κσ ~ 2.0).
a, The T-dependence of the roughness h, thickness l and l/h. The interface is rough and thick at all T (h ~ 4.6d, l ~ 3.5d), At each panel, the grey line indicates the average value. b, The T-dependence of the solid surface thickness ls, interface liquid thickness l − ls and ls/(l − ls). c, The T-dependence of flayered (fraction of layered particle at interface), funlayered (fraction of unlayered particle at interface) and fico (fraction of icosahedron-like particle in bulk layers). This rough, thick and layered nature of the interface is little affected by the increasing amounts of icosahedron-like structures in the bulk due to deeper supercooling.
Extended Data Fig. 5 The island-growth mode of crystal for rough and thick interface at other κσ and T in experiments and simulations.
a, Contour plot of the height of the crystal front h(x, y), La(x, y), vver(x, y) and vlat(x, y) (from top to bottom respectively, κσ ~ 3.2, T ~ 0.4Tm in experiments. b, c The same quantities as in a for κσ ~ 2.0, T ~ 0.6Tm in experiments (b) and κσ ~ 2.0, T ~ 0.9Tm in simulations (c). At all κσ and T, we observe the vertical growth mode generating new islands and lateral growth mode around the island. The icosahedron-like particles at the interface (white spheres) is mainly eliminated by the lateral growth in a. Icosahedron-like structures are less frequent in higher-T samples and thus have little effect on the growth mode in c.
Extended Data Fig. 6 The collective ordering process determined by the interface thickness.
a, The evolution of \({\overline{q}}_{6}\) for one particle layer with respect to time t (κσ ~ 2.0, T ~ 0.1Tm in simulations). The time period Δt (in between red lines) corresponds to a propagation length 4d of the growth front. b, The interface profile \({\overline{q}}_{6}(z)\) with respect to z/d at t (top) and t + Δt (bottom). The propagation length during Δt is illustrated by the distance between the two grey lines. c, The interface profile La(z) with respect to z/d at t (top) and t + Δt (bottom). It supports a collective crystal growth process with the crystal growth speed expressed as:v = l/Δt.
Extended Data Fig. 7 Definition of the preordering and the further ordering based on the \({L}_{a}-{\overline{q}}_{6}\) correlation.
a, b, The \({L}_{a}-{\overline{q}}_{6}\) correlation at κσ ~ 2.0, T ~ 0.6Tm in experiments (a) and simulations (b). c, d, The \({L}_{a}-{\overline{q}}_{6}\) correlation at κσ ~ 3.2, T ~ 0.4Tm in experiments (c) and simulations (d). e,f, The \({L}_{a}-{\overline{q}}_{6}\) correlation at κσ ~ 5.0, T ~ 0.5Tm in experiments (e) and simulations (f). The fast increase of \({\overline{q}}_{6}\) (from the left side of the unlayered liquid part, La ~ 0.14, to the solid-liquid boundary, \({\overline{q}}_{6} \sim {\overline{q}}_{6}^{\star }\)) corresponds to the preordering process, whereas the further slow growth of \({\overline{q}}_{6}\) inside the solid phase (from \({\overline{q}}_{6} \sim {\overline{q}}_{6}^{\star }\) to \({\overline{q}}_{6} \sim {\overline{q}}_{6}^{F}\)) corresponds to the repairing of the ‘quenched-in disorder’. The error bars represent the standard deviation of the \({\overline{q}}_{6}\) values corresponding to the values of La within a small binsize (the symbol-symbol distance on x axis).
Extended Data Fig. 8 The correlation of l and τave with the choice of \({\overline{q}}_{6}^{\star }\).
a,l versus τave with different choice of \({\overline{q}}_{6}^{\star }\). b, the \({\overline{q}}_{6}^{\star }\)-dependence of l (top) and τave (bottom). Although the selection of the threshold value of \({\overline{q}}_{6}^{\star }\) weakly modifies the values of l and τave, it affects them proportionally, and thus produces little change in l/τave. Such proportionality between the length scale of collective motion and its time scale is a genuine feature of diffusionless collective rearrangements.
Extended Data Fig. 9 Diffusive motion and the comparison with diffusionless ordering model.
a, The time evolution of the mean-square displacement Δr(t)2 for final crystals, initial crystals, and unlayered liquid in experiments (κσ ~ 3.2, T ~ 0.4Tm). We illustrate τave and τα by vertical green and orange lines, respectively. b, The time evolution of the mean-square displacement Δr(t)2 for bulk samples at various T in simulations (κσ ~ 2.0). we illustrate τave by vertical green line. The subdiffusive behavior becomes obvious at deep supercoolings. c, The diffusive constant determined in b and the comparison between diffusive and diffusionless models. Diffusive constant D (top) is determined as \({{\Delta }}\,r{({\tau }_{{\rm{ave}}})}^{2}/6{\tau }_{{\rm{ave}}}\).
Extended Data Fig. 10 The comparison of low-ϕ and high-ϕ crystal growth at deep supercooling in experiments.
a, The continuous fast crystal growth at ϕ = 20 %, where the interaction is soft-repulsive. The fast crystal growth modes can propagate more than 40 particle layers. b, Crystal growth for ϕ = 35 % sample, where the hard-core effect is prominent. Crystal growth becomes slower and slower as the accumulation of trapped-in defects. There is no homogeneous nucleation behavior observed inside the sample.
Supplementary information
Supplementary Information
Supplementary Figs.1–14 and Discussion.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 9
Statistical source data.
Source Data Extended Data Fig. 10
Statistical source data.
Rights and permissions
About this article
Cite this article
Gao, Q., Ai, J., Tang, S. et al. Fast crystal growth at ultra-low temperatures. Nat. Mater. 20, 1431–1439 (2021). https://doi.org/10.1038/s41563-021-00993-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41563-021-00993-6
This article is cited by
-
Microscopic mechanisms of pressure-induced amorphous-amorphous transitions and crystallisation in silicon
Nature Communications (2024)
-
Optofluidic crystallithography for directed growth of single-crystalline halide perovskites
Nature Communications (2024)
-
Diffusion metamaterials
Nature Reviews Physics (2023)
-
Spatiotemporal observation of quantum crystallization of electrons
Nature Communications (2023)
-
Fast crystal growth of ice VII owing to the decoupling of translational and rotational ordering
Communications Physics (2023)