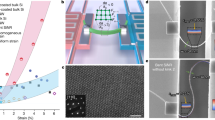
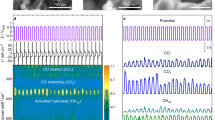
Abstract
Understanding how the bulk structure of a material affects catalysis on its surface is critical to the development of actionable catalyst design principles. Bulk defects have been shown to affect electrocatalytic materials that are important for energy conversion systems, but the structural origins of these effects have not been fully elucidated. Here we use a combination of high-resolution scanning electrochemical cell microscopy and electron backscatter diffraction to visualize the potential-dependent electrocatalytic carbon dioxide \(({\mathrm{C}}{\mathrm{O}}_{2})\) electroreduction and hydrogen \(({{\mathrm{H}}_{2}})\) evolution activity on Au electrodes and probe the effects of bulk defects. Comparing colocated activity maps and videos to the underlying microstructure and lattice deformation supports a model in which CO2 electroreduction is selectively enhanced by surface-terminating dislocations, which can accumulate at grain boundaries and slip bands. Our results suggest that the deliberate introduction of dislocations into materials is a promising strategy for improving catalytic properties.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The authors declare that all data supporting the findings of this study are included within the paper and its Supplementary Information files. Source data are available from the corresponding authors upon reasonable request.
References
Feng, X., Jiang, K., Fan, S. & Kanan, M. W. Grain-boundary-dependent CO2 electroreduction activity. J. Am. Chem. Soc. 137, 4606–4609 (2015).
Feng, X., Jiang, K., Fan, S. & Kanan, M. W. A direct grain-boundary-activity correlation for CO electroreduction on Cu nanoparticles. ACS Cent. Sci. 2, 169–174 (2016).
Mariano, R. G., McKelvey, K., White, H. S. & Kanan, M. W. Selective increase in CO2 electroreduction activity at grain-boundary surface terminations. Science 358, 1187–1192 (2017).
Verdaguer-Casadevall, A. et al. Probing the active surface sites for CO reduction on oxide-derived copper electrocatalysts. J. Am. Chem. Soc. 137, 9808–9811 (2015).
Lee, S. H. et al. Correlating oxidation state and surface area to activity from operando studies of copper CO electroreduction catalysts in a gas-fed device. ACS Catal. 10, 8000–8011 (2020).
Xu, Z. et al. Dynamic restructuring induced Cu nanoparticles with ideal nanostructure for selective multi-carbon compounds production via carbon dioxide electroreduction. J. Catal. 383, 42–50 (2020).
Humphreys, F. J. A unified theory of recovery, recrystallization and grain growth, based on the stability and growth of cellular microstructures—I. The basic model. Acta Mater. 45, 4231–4240 (1997).
Humphreys, J., Rohrer, G. S. & Rollett, A. in Recrystallization and Related Annealing Phenomena 3rd edn, 145–197 (Elsevier, 2017).
Engbæk, J., Schiøtz, J., Dahl-Madsen, B. & Horch, S. Atomic structure of screw dislocations intersecting the Au(111) surface: a combined scanning tunneling microscopy and molecular dynamics study. Phys. Rev. B 74, 195434 (2006).
Chidsey, C. E. D., Loiacono, D. N., Sleator, T. & Nakahara, S. STM study of the surface morphology of gold on mica. Surf. Sci. 200, 45–66 (1988).
Radetic, T., Lançon, F. & Dahmen, U. Chevron defect at the intersection of grain boundaries with free surfaces in Au. Phys. Rev. Lett. 89, 085502 (2002).
Giela, T., Freindl, K., Spiridis, N. & Korecki, J. Au(111) films on W(110) studied by STM and LEED – uniaxial reconstruction, dislocations and Ag nanostructures. Appl. Surf. Sci. 312, 91–96 (2014).
Morgenstern, K., Lægsgaard, E. & Besenbacher, F. STM study of step dynamics around a bulk dislocation intersection with a Ag(111) surface. Phys. Rev. B 71, 155426 (2005).
Christiansen, J. et al. Atomic-scale structure of dislocations revealed by scanning tunneling microscopy and molecular dynamics. Phys. Rev. Lett. 88, 206106 (2002).
Barth, J. V., Brune, H., Ertl, G. & Behm, R. J. Scanning tunneling microscopy observations on the reconstructed Au(111) surface: atomic structure, long-range superstructure, rotational domains, and surface defects. Phys. Rev. B 42, 9307–9318 (1990).
Trevor, D. J., Chidsey, C. E. D. & Loiacono, D. N. In situ scanning-tunneling-microscope observation of roughening, annealing, and dissolution of gold (111) in an electrochemical cell. Phys. Rev. Lett. 62, 929–932 (1989).
Grim, R. G. et al. Transforming the carbon economy: challenges and opportunities in the convergence of low-cost electricity and reductive CO2 utilization. Energy Environ. Sci. 13, 472–494 (2020).
Hori, Y., Kikuchi, K. & Suzuki, S. Production of CO and CH4 in electrochemical reduction of CO2 at metal electrodes in aqueous hydrogencarbonate solution. Chem. Lett. 14, 1695–1698 (1985).
Bentley, C. L., Kang, M. & Unwin, P. R. Nanoscale surface structure–activity in electrochemistry and electrocatalysis. J. Am. Chem. Soc. 141, 2179–2193 (2019).
Aaronson, B. D. B. et al. Pseudo-single-crystal electrochemistry on polycrystalline electrodes: visualizing activity at grains and grain boundaries on platinum for the Fe2+/Fe3+ redox reaction. J. Am. Chem. Soc. 135, 3873–3880 (2013).
Wang, Y., Gordon, E. & Ren, H. Mapping the nucleation of H2 bubbles on polycrystalline Pt via scanning electrochemical cell microscopy. J. Phys. Chem. Lett. 10, 3887–3892 (2019).
Kang, M., Momotenko, D., Page, A., Perry, D. & Unwin, P. R. Frontiers in nanoscale electrochemical imaging: faster, multifunctional, and ultrasensitive. Langmuir 32, 7993–8008 (2016).
Ornelas, I. M., Unwin, P. R. & Bentley, C. L. High-throughput correlative electrochemistry–microscopy at a transmission electron microscopy grid electrode. Anal. Chem. 91, 14854–14859 (2019).
Bentley, C. L., Kang, M. & Unwin, P. R. Nanoscale structure dynamics within electrocatalytic materials. J. Am. Chem. Soc. 139, 16813–16821 (2017).
Wahab, O. J., Kang, M. & Unwin, P. R. Scanning electrochemical cell microscopy: a natural technique for single entity electrochemistry. Curr. Opin. Electrochem. 22, 120–128 (2020).
Ebejer, N. et al. Scanning electrochemical cell microscopy: a versatile technique for nanoscale electrochemistry and functional imaging. Annu. Rev. Anal. Chem. 6, 329–351 (2013).
Bentley, C. L. et al. Electrochemical maps and movies of the hydrogen evolution reaction on natural crystals of molybdenite (MoS2): basal vs. edge plane activity. Chem. Sci. 8, 6583–6593 (2017).
Ripatti, D. S., Veltman, T. R. & Kanan, M. W. Carbon monoxide gas diffusion electrolysis that produces concentrated C2 products with high single-pass conversion. Joule 3, 240–256 (2019).
Ringe, S. et al. Double layer charging driven carbon dioxide adsorption limits the rate of electrochemical carbon dioxide reduction on gold. Nat. Commun. 11, 33 (2020).
Wuttig, A., Yaguchi, M., Motobayashi, K., Osawa, M. & Surendranath, Y. Inhibited proton transfer enhances Au-catalyzed CO2-to-fuels selectivity. Proc. Natl Acad. Sci. USA 113, E4585–E4593 (2016).
Zhang, B. A., Ozel, T., Elias, J. S., Costentin, C. & Nocera, D. G. Interplay of homogeneous reactions, mass transport, and kinetics in determining selectivity of the reduction of CO2 on gold electrodes. ACS Cent. Sci. 5, 1097–1105 (2019).
Wuttig, A., Yoon, Y., Ryu, J. & Surendranath, Y. Bicarbonate is not a general acid in Au-catalyzed CO2 electroreduction. J. Am. Chem. Soc. 139, 17109–17113 (2017).
Ooka, H., Figueiredo, M. C. & Koper, M. T. M. Competition between hydrogen evolution and carbon dioxide reduction on copper electrodes in mildly acidic media. Langmuir 33, 9307–9313 (2017).
Hall, A. S., Yoon, Y., Wuttig, A. & Surendranath, Y. Mesostructure-induced selectivity in CO2 reduction catalysis. J. Am. Chem. Soc. 137, 14834–14837 (2015).
Mezzavilla, S., Horch, S., Stephens, I. E. L., Seger, B. & Chorkendorff, I. Structure sensitivity in the electrocatalytic reduction of CO2 with gold catalysts. Angew. Chem. Int. Ed. 131, 3814–3818 (2019).
Cai, W. & Nix, W. D. Imperfections in Crystalline Solids (Cambridge Univ. Press, 2016).
Britton, T. B., Holton, I., Meaden, G. & Dingley, D. High angular resolution electron backscatter diffraction: measurement of strain in functional and structural materials. Microsc. Anal. 27, 8–13 (2013).
Wilkinson, A. J. & Randman, D. Determination of elastic strain fields and geometrically necessary dislocation distributions near nanoindents using electron back scatter diffraction. Phil. Mag. 90, 1159–1177 (2010).
Dingley, D. J., Wilkinson, A. J., Meaden, G. & Karamched, P. S. Elastic strain tensor measurement using electron backscatter diffraction in the SEM. J. Electron Microsc. 59, S155–S163 (2010).
Wilkinson, A. J., Meaden, G. & Dingley, D. J. Mapping strains at the nanoscale using electron back scatter diffraction. Superlattices Microstruct. 45, 285–294 (2009).
Jiang, J., Britton, T. B. & Wilkinson, A. J. Evolution of dislocation density distributions in copper during tensile deformation. Acta Mater. 61, 7227–7239 (2013).
Guo, Y., Britton, T. B. & Wilkinson, A. J. Slip band–grain boundary interactions in commercial-purity titanium. Acta Mater. 76, 1–12 (2014).
Littlewood, P. D., Britton, T. B. & Wilkinson, A. J. Geometrically necessary dislocation density distributions in Ti-6Al-4V deformed in tension. Acta Mater. 59, 6489–6500 (2011).
Wallis, D., Hansen, L. N., Britton, T. B. & Wilkinson, A. J. Geometrically necessary dislocation densities in olivine obtained using high-angular resolution electron backscatter diffraction. Ultramicroscopy 168, 34–45 (2016).
Britton, T. B., Liang, H., Dunne, F. P. E. & Wilkinson, A. J. The effect of crystal orientation on the indentation response of commercially pure titanium: experiments and simulations. Proc. R. Soc. A 466, 695–719 (2010).
Britton, T. B. & Wilkinson, A. J. High resolution electron backscatter diffraction measurements of elastic strain variations in the presence of larger lattice rotations. Ultramicroscopy 114, 82–95 (2012).
Ulvestad, A., Clark, J. N., Harder, R., Robinson, I. K. & Shpyrko, O. G. 3D imaging of twin domain defects in gold nanoparticles. Nano Lett. 15, 4066–4070 (2015).
Yau, A., Cha, W., Kanan, M. W., Stephenson, G. B. & Ulvestad, A. Bragg coherent diffractive imaging of single-grain defect dynamics in polycrystalline films. Science 356, 739–742 (2017).
Yu, H., Liu, J., Karamched, P., Wilkinson, A. J. & Hofmann, F. Mapping the full lattice strain tensor of a single dislocation by high angular resolution transmission Kikuchi diffraction (HR-TKD). Scr. Mater. 164, 36–41 (2019).
Wallis, D., Hansen, L. N., Britton, T. B. & Wilkinson, A. J. High‐angular resolution electron backscatter diffraction as a new tool for mapping lattice distortion in geological minerals. J. Geophys. Res. Solid Earth 124, 6337–6358 (2019).
Humphreys, F. J. Grain and subgrain characterisation by electron backscatter diffraction. J. Mater. Sci. 36, 3833–3854 (2001).
Liu, M. et al. Enhanced electrocatalytic CO2 reduction via field-induced reagent concentration. Nature 537, 382–386 (2016).
Wang, Y. & Hall, A. S. Pulsed electrodeposition of metastable Pd31Bi12 nanoparticles for oxygen reduction electrocatalysis. ACS Energy Lett. 5, 17–22 (2020).
Hamelin, A. Cyclic voltammetry at gold single-crystal surfaces. Part 1. Behaviour at low-index faces. J. Electroanal. Chem. 407, 1–11 (1996).
Chen, C.-H. et al. Voltammetric scanning electrochemical cell microscopy: dynamic imaging of hydrazine electro-oxidation on platinum electrodes. Anal. Chem. 87, 5782–5789 (2015).
Acknowledgements
Work at Stanford was supported by the National Science Foundation (CHE-1855950). R.G.M. gratefully acknowledges Stanford University for a DARE fellowship and J.A.R. gratefully acknowledges a Stanford Graduate Fellowship. M.K. and P.R.U. are grateful to the Warwick–Monash Accelerator Fund for support. M.K. also acknowledges support from the Leverhulme Trust for an Early Career Fellowship. I.J.M. and P.R.U. are supported by Engineering and Physical Sciences Research Council Programme Grant EP/R018820/1. P.R.U. thanks the Royal Society for a Wolfson Research Merit Award. O.J.W. acknowledges support from the University of Warwick Chancellor’s International Scholarship. Parts of this work were performed at the Stanford Nano Shared Facilities, which is supported by the National Science Foundation under award ECCS-1542152.
Author information
Authors and Affiliations
Contributions
R.G.M., M.K., P.R.U. and M.W.K. conceived and designed the study. R.G.M., M.K. and O.J.W. performed SECCM experiments. O.J.W. prepared Au samples for SECCM imaging. R.G.M. and J.A.R. performed gas diffusion electrode electrolysis studies. R.G.M., M.K. and O.J.W. performed SEM/EBSD imaging of the samples, and R.G.M. performed HR-EBSD measurements and analysis. I.J.M. and P.R.U. designed the finite element method calculations, and I.J.M. built the COMSOL model. R.G.M., M.K. and M.W.K. wrote the initial draft of the paper, and all authors contributed to the final version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Materials thanks the anonymous reviewers for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–13, Tables 1–5, Notes 1 and 2, captions for Videos 1–5 and references.
Supplementary Video 1
Spatially resolved electrochemical video (707 pixels over a 49 μm × 7.7 μm scan area, 220 image frames) obtained with the voltammetric SECCM protocol, visualizing activity of H2 evolution on Sample A.
Supplementary Video 2
Spatially resolved electrochemical video (1,140 pixels over a 30 μm × 9.5 μm scan area, 220 image frames) obtained with the voltammetric SECCM protocol, visualizing activity of CO2 electroreduction on Sample A.
Supplementary Video 3
Spatially resolved electrochemical video (369 pixels over a 20.5 μm × 4.5 μm scan area, 220 image frames) obtained with the voltammetric SECCM protocol, visualizing activity of H2 evolution on Sample A.
Supplementary Video 4
Spatially resolved electrochemical video (1,281 pixels over a 30.5 μm × 10.5 μm scan area, 220 image frames) obtained with the voltammetric SECCM protocol, visualizing activity of CO2 electroreduction on Sample A.
Supplementary Video 5
Spatially resolved electrochemical video (756 pixels over a 13.5 μm × 14 μm scan area, 220 image frames) obtained with the voltammetric SECCM protocol, visualizing activity of CO2 electroreduction on Sample B.
Rights and permissions
About this article
Cite this article
Mariano, R.G., Kang, M., Wahab, O.J. et al. Microstructural origin of locally enhanced CO2 electroreduction activity on gold. Nat. Mater. 20, 1000–1006 (2021). https://doi.org/10.1038/s41563-021-00958-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41563-021-00958-9
This article is cited by
-
Correlating activities and defects in (photo)electrocatalysts using in-situ multi-modal microscopic imaging
Nature Communications (2024)
-
Pure-water-fed, electrocatalytic CO2 reduction to ethylene beyond 1,000 h stability at 10 A
Nature Energy (2024)
-
Nanostructured catalysts for CO2 reduction: systematic insights and emerging strategies
Research on Chemical Intermediates (2024)
-
A critical review of operating stability issues in electrochemical CO2 reduction
Science China Materials (2024)
-
Pb induced dislocation defects of PtCo systems: Strain-triggered oxygen reduction reaction for PEMFC
Nano Research (2024)