Abstract
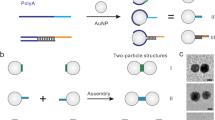
Nature has evolved strategies to encode information within a single biopolymer to program biomolecular interactions with characteristic stoichiometry, orthogonality and reconfigurability. Nevertheless, synthetic approaches for programming molecular reactions or assembly generally rely on the use of multiple polymer chains (for example, patchy particles). Here we demonstrate a method for patterning colloidal gold nanoparticles with valence bond analogues using single-stranded DNA encoders containing polyadenine (polyA). By programming the order, length and sequence of each encoder with alternating polyA/non-polyA domains, we synthesize programmable atom-like nanoparticles (PANs) with n-valence that can be used to assemble a spectrum of low-coordination colloidal molecules with different composition, size, chirality and linearity. Moreover, by exploiting the reconfigurability of PANs, we demonstrate dynamic colloidal bond-breaking and bond-formation reactions, structural rearrangement and even the implementation of Boolean logic operations. This approach may be useful for generating responsive functional materials for distinct technological applications.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All the data that support the findings of this study are available within the paper and its Supplementary Information files, and from the corresponding authors upon reasonable request.
References
Garzoni, M., Okuro, K., Ishii, N., Aida, T. & Pavan, G. M. Structure and shape effects of molecular glue on supramolecular tubulin assemblies. ACS Nano 8, 904–914 (2014).
Bednar, J. et al. Nucleosomes, linker DNA, and linker histone form a unique structural motif that directs the higher-order folding and compaction of chromatin. Proc. Natl Acad. Sci. USA 95, 14173–14178 (1998).
Lane, T., Serwer, P., Hayes, S. J. & Eiserling, F. Quantized viral-DNA packaging revealed by rotating gel-electrophoresis. Virology 174, 472–478 (1990).
Routh, A., Sandin, S. & Rhodes, D. Nucleosome repeat length and linker histone stoichiometry determine chromatin fiber structure. Proc. Natl Acad. Sci. USA 105, 8872–8877 (2008).
Folsch, S., Martinez-Blanco, J., Yang, J. S., Kanisawa, K. & Erwin, S. C. Quantum dots with single-atom precision. Nat. Nanotechnol. 9, 505–508 (2014).
Chen, J. W. et al. Artificial muscle-like function from hierarchical supramolecular assembly of photoresponsive molecular motors. Nat. Chem. 10, 132–138 (2018).
Zhang, C. et al. A general approach to DNA-programmable atom equivalents. Nat. Mater. 12, 741–746 (2013).
Macfarlane, R. J., O’Brien, M. N., Petrosko, S. H. & Mirkin, C. A. Nucleic acid-modified nanostructures as programmable atom equivalents: forging a new “Table of Elements”. Angew. Chem. Int. Edit. 52, 5688–5698 (2013).
Jones, M. R. et al. DNA-nanoparticle superlattices formed from anisotropic building blocks. Nat. Mater. 9, 913–917 (2010).
Wang, Y. et al. Colloids with valence and specific directional bonding. Nature 491, 51–55 (2012).
Kraft, D. J. et al. Surface roughness directed self-assembly of patchy particles into colloidal micelles. Proc. Natl Acad. Sci. USA 109, 10787–10792 (2012).
Feng, L., Dreyfus, R., Sha, R. J., Seeman, N. C. & Chaikin, P. M. DNA patchy particles. Adv. Mater. 25, 2779–2783 (2013).
Groschel, A. H. et al. Guided hierarchical co-assembly of soft patchy nanoparticles. Nature 503, 247–251 (2013).
Rozynek, Z., Mikkelsen, A., Dommersnes, P. & Fossum, J. O. Electroformation of Janus and patchy capsules. Nat. Commun. 5, 3945 (2014).
Newton, A. C., Groenewold, J., Kegel, W. K. & Bolhuis, P. G. Rotational diffusion affects the dynamical self-assembly pathways of patchy particles. Proc. Natl Acad. Sci. USA 112, 15308–15313 (2015).
Gong, Z., Hueckel, T., Yi, G. R. & Sacanna, S. Patchy particles made by colloidal fusion. Nature 550, 234–238 (2017).
Choueiri, R. M. et al. Surface patterning of nanoparticles with polymer patches. Nature 538, 79–83 (2016).
Jones, M. R., Seeman, N. C. & Mirkin, C. A. Programmable materials and the nature of the DNA bond. Science 347, 1260901 (2015).
Tan, S. J., Campolongo, M. J., Luo, D. & Cheng, W. Building plasmonic nanostructures with DNA. Nat. Nanotechnol. 6, 268–276 (2011).
Liu, W. Y., Halverson, J., Tian, Y., Tkachenko, A. V. & Gang, O. Self-organized architectures from assorted DNA-framed nanoparticles. Nat. Chem. 8, 867–873 (2016).
Li, Y., Liu, Z., Yu, G., Jiang, W. & Mao, C. Self-assembly of molecule-like nanoparticle clusters directed by DNA nanocages. J. Am. Chem. Soc. 137, 4320–4323 (2015).
Alivisatos, A. P. et al. Organization of ‘nanocrystal molecules’ using DNA. Nature 382, 609–611 (1996).
Park, S. Y. et al. DNA-programmable nanoparticle crystallization. Nature 451, 553–556 (2008).
Macfarlane, R. J. et al. Nanoparticle superlattice engineering with DNA. Science 334, 204–208 (2011).
Auyeung, E. et al. DNA-mediated nanoparticle crystallization into wulff polyhedra. Nature 505, 73–77 (2014).
Liu, W. et al. Diamond family of nanoparticle superlattices. Science 351, 582–586 (2016).
Tan, L. H., Xing, H., Chen, H. Y. & Lu, Y. Facile and efficient preparation of anisotropic DNA-functionalized gold nanoparticles and their regioselective assembly. J. Am. Chem. Soc. 135, 17675–17678 (2013).
Xing, H. et al. Bottom-up strategy to prepare nanoparticles with a single DNA strand. J. Am. Chem. Soc. 139, 3623–3626 (2017).
Ben Zion, M. Y. et al. Self-assembled three-dimensional chiral colloidal architecture. Science 358, 633–636 (2017).
Zhang, Y. et al. Transfer of two-dimensional oligonucleotide patterns onto stereocontrolled plasmonic nanostructures through DNA-origami-based nanoimprinting lithography. Angew. Chem. Int. Edit. 55, 8036–8040 (2016).
Edwardson, T. G. W., Lau, K. L., Bousmail, D., Serpell, C. J. & Sleiman, H. F. Transfer of molecular recognition information from DNA nanostructures to gold nanoparticles. Nat. Chem. 8, 162–170 (2016).
Xu, X., Rosi, N. L., Wang, Y., Huo, F. & Mirkin, C. A. Asymmetric functionalization of gold nanoparticles with oligonucleotides. J. Am. Chem. Soc. 128, 9286–9287 (2006).
Huo, F., Lytton-Jean, A. K. R. & Mirkin, C. A. Asymmetric functionalization of nanoparticles based on thermally addressable DNA interconnects. Adv. Mater. 18, 2304–2306 (2006).
Zhang, Y. et al. Selective transformations between nanoparticle superlattices via the reprogramming of DNA-mediated interactions. Nat. Mater. 14, 840–847 (2015).
Kim, Y., Macfarlane, R. J., Jones, M. R. & Mirkin, C. A. Transmutable nanoparticles with reconfigurable surface ligands. Science 351, 579–582 (2016).
Pei, H. et al. Designed diblock oligonucleotide for the synthesis of spatially isolated and highly hybridizable functionalization of DNA-gold nanoparticle banoconjugates. J. Am. Chem. Soc. 134, 11876–11879 (2012).
Yao, G. et al. Clicking DNA to gold nanoparticles: poly-adenine-mediated formation of monovalent DNA-gold nanoparticle conjugates with nearly quantitative yield. NPG Asia Mater. 7, e159 (2015).
Fan, J. A. et al. Self-assembled plasmonic nanoparticle clusters. Science 328, 1135–1138 (2010).
Hentschel, M., Schäferling, M., Duan, X., Giessen, H. & Liu, N. Chiral plasmonics. Sci. Adv. 3, e1602735 (2017).
Soloveichik, D., Seelig, G. & Winfree, E. DNA as a universal substrate for chemical kinetics. Proc. Natl Acad. Sci. USA 107, 5393–5398 (2010).
Zhang, D. Y. & Winfree, E. Control of DNA strand displacement kinetics using toehold exchange. J. Am. Chem. Soc. 131, 17303–17314 (2009).
Kotani, S. & Hughes, W. L. Multi-arm junctions for dynamic DNA nanotechnology. J. Am. Chem. Soc. 139, 6363–6368 (2017).
Liu, D. B. et al. Resettable, multi-readout logic gates based on controllably reversible aggregation of gold nanoparticles. Angew. Chem. Int. Edit. 50, 4103–4107 (2011).
Feringa, B. L. The art of building small: from molecular switches to motors (Nobel Lecture). Angew. Chem. Int. Edit. 56, 11060–11078 (2017).
Bruns, C. J. & Stoddart, J. F. The Nature of the Mechanical Bond: from Molecules to Machines (John Wiley & Sons, 2017).
Balzani, V., Credi, A. & Venturi, M. Molecular Devices and Machines – A Journey into the Nano World (Wiley-VCH, 2003).
Shen, J. et al. Valence-engineering of quantum dots using programmable DNA scaffolds. Angew. Chem. Int. Edit. 56, 16077–16081 (2017).
Zhu, D. et al. Coordination-mediated programmable assembly of unmodified oligonucleotides on plasmonic silver nanoparticles. ACS Appl. Mater. Inter. 7, 11047–11052 (2015).
Zhou, L. et al. DNA-mediated construction of hollow upconversion nanoparticles for protein harvesting and near-infrared light triggered release. Adv. Mater. 26, 2424–2430 (2014).
Cecconello, A., Besteiro, L. V., Govorov, A. O. & Willner, I. Chiroplasmonic DNA-based nanostructures. Nat. Rev. Mater 2, 17039 (2017).
Luk’yanchuk, B. et al. The Fano resonance in plasmonic nanostructures and metamaterials. Nat. Mater. 9, 707–715 (2010).
Urban, M. J. et al. Plasmonic toroidal metamolecules assembled by DNA origami. J. Am. Chem. Soc. 138, 5495–5498 (2016).
Lim, D. K. et al. Highly uniform and reproducible surface-enhanced Raman scattering from DNA-tailorable nanoparticles with 1-nm interior gap. Nat. Nanotechnol. 6, 452–460 (2011).
Hartl, C. et al. Position accuracy of gold nanoparticles on DNA origami structures studied with small-angle X-ray scattering. Nano Lett. 18, 2609–2615 (2018).
Nielsen, S. S. et al. BioXTAS RAW, a software program for high-throughput automated small-angle X-ray scattering data reduction and preliminary analysis. J. Appl. Crystallogr. 42, 959–964 (2009).
M. Doucet et al. SasView v.4.1.2 (SasView, 2017); https://doi.org/10.5281/zenodo.825675.
Dobrynin, A. V., Rubinstein, M. & Obukhov, S. P. Cascade of transitions of polyelectrolytes in poor solvents. Macromolecules 29, 2974–2979 (1996).
Case, D. A. et al. The amber biomolecular simulation programs. J. Comput. Chem. 26, 1668–1688 (2005).
Zgarbova, M. et al. Refinement of the sugar-phosphate backbone torsion beta for AMBER force fields improves the description of Z- and B-DNA. J. Chem. Theory Comput. 11, 5723–5736 (2015).
Heinz, H., Lin, T. J., Mishra, R. K. & Emami, F. S. Thermodynamically consistent force fields for the assembly of inorganic, organic, and biological nanostructures: the INTERFACE force field. Langmuir 29, 1754–1765 (2013).
Jorgensen, W. L., Chandrasekhar, J., Madura, J. D., Impey, R. W. & Klein, M. L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 79, 926–935 (1983).
Tuckerman, M. E., Liu, Y., Ciccotti, G. & Martyna, G. J. Non-Hamiltonian molecular dynamics: generalizing Hamiltonian phase space principles to non-Hamiltonian systems. J. Chem. Phys. 115, 1678–1702 (2001).
Acknowledgements
This work was financially supported by the National Key R&D Program of China (2016YFA0201200), National Natural Science Foundation of China (21834007, 21675167, 31571014, U1532119, 21775157, 11575278), the National Science Foundation (1531991), China Postdoctoral Science Foundation (2015M580373, 2016T90396), the Open Large Infrastructure Research of the Chinese Academy of Sciences, the LU JIAXI International team programme supported by CAS and the K.C. Wong Education Foundation, Shanghai Jiao Tong University. The authors are also thankful for the staff from BL19U2 beamline of National Facility for Protein Science Shanghai (NFPS) at Shanghai Synchrotron Radiation Facility (SSRF) for assistance during data collection.
Author information
Authors and Affiliations
Contributions
C.F. and B.L.F. directed the research. C.F., G.Y. and J.L. conceived the study. G.Y., Q.L. and X.C. performed experiments. R.P.N., D.W., G.Y. and H.Y. performed cryogenic electron microscopy imaging and analysis. F.W. and H.P. performed CD theoretical calculations. Z.Q. performed MD simulations. X.L. and X.C. performed SAXS experiments. Z.G. and H.P. assisted in the preparation of polyA–AuNPs. X.L., X.Z. and L.W. assisted with the TEM imaging. G.Y., J.L., Q.L., B.L.F. and C.F. analysed data and wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figures 1–30 and Tables 1 and 2.
Rights and permissions
About this article
Cite this article
Yao, G., Li, J., Li, Q. et al. Programming nanoparticle valence bonds with single-stranded DNA encoders. Nat. Mater. 19, 781–788 (2020). https://doi.org/10.1038/s41563-019-0549-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41563-019-0549-3
This article is cited by
-
DNA as a universal chemical substrate for computing and data storage
Nature Reviews Chemistry (2024)
-
DNA framework-engineered chimeras platform enables selectively targeted protein degradation
Nature Communications (2023)
-
DNA-framework-based multidimensional molecular classifiers for cancer diagnosis
Nature Nanotechnology (2023)
-
Nanoscale 3D spatial addressing and valence control of quantum dots using wireframe DNA origami
Nature Communications (2022)
-
Coumarin 6H-fused fluorescent probe for highly sensitive detection of coralyne using oligonucleotide-modified silver nanoparticles
Analytical and Bioanalytical Chemistry (2022)