Abstract
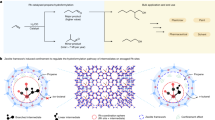
The aqueous electrocatalytic reduction of CO2 into alcohol and hydrocarbon fuels presents a sustainable route towards energy-rich chemical feedstocks. Cu is the only material able to catalyse the substantial formation of multicarbon products (C2/C3), but competing proton reduction to hydrogen is an ever-present drain on selectivity. Here, a superhydrophobic surface was generated by 1-octadecanethiol treatment of hierarchically structured Cu dendrites, inspired by the structure of gas-trapping cuticles on subaquatic spiders. The hydrophobic electrode attained a 56% Faradaic efficiency for ethylene and 17% for ethanol production at neutral pH, compared to 9% and 4% on a hydrophilic, wettable equivalent. These observations are assigned to trapped gases at the hydrophobic Cu surface, which increase the concentration of CO2 at the electrode–solution interface and consequently increase CO2 reduction selectivity. Hydrophobicity is thus proposed as a governing factor in CO2 reduction selectivity and can help explain trends seen on previously reported electrocatalysts.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Raw data used in preparation of this manuscript is available to download at https://doi.org/10.7910/DVN/DSPZHE.
References
IPCC Climate Change 2013: The Physical Science Basis (eds Stocker, T. F. et al.) (Cambridge Univ. Press, 2013).
Raciti, D. & Wang, C. Recent advances in CO2 reduction electrocatalysis on copper. ACS Energy Lett. 3, 1545–1556 (2018).
Qiao, J., Liu, Y., Hong, F. & Zhang, J. A review of catalysts for the electroreduction of carbon dioxide to produce low-carbon fuels. Chem. Soc. Rev. 43, 631–675 (2014).
Sander, R. Compilation of Henry’s law constants, version 3.99. Atmos. Chem. Phys. Discuss. 14, 29615–30521 (2014).
Hori, Y., Kikuchi, K. & Suzuki, S. Production of CO and CH4 in electrochemical reduction of CO2 at metal electrodes in aqueous hydrogencarbonate solution. Chem. Lett. 14, 1695–1698 (1985).
Wang, J., Zhang, F., Kang, X. & Chen, S. Organic functionalization of metal catalysts: enhanced activity towards electroreduction of carbon dioxide. Curr. Opin. Electrochem. 13, 40–46 (2019).
Zhou, Y. et al. Dopant-induced electron localization drives CO2 reduction to C2 hydrocarbons. Nat. Chem. 10, 974–980 (2018).
Varela, A. S., Kroschel, M., Reier, T. & Strasser, P. Controlling the selectivity of CO2 electroreduction on copper: the effect of the electrolyte concentration and the importance of the local pH. Catal. Today 260, 8–13 (2016).
Piontek, S. et al. Bio-inspired design: bulk iron–nickel sulfide allows for efficient solvent-dependent CO2 reduction. Chem. Sci. 10, 1075–1081 (2019).
Li, Y. et al. Structure-sensitive CO2 electroreduction to hydrocarbons on ultrathin 5-fold twinned copper nanowires. Nano Lett. 17, 1312–1317 (2017).
De Luna, P. et al. Catalyst electro-redeposition controls morphology and oxidation state for selective carbon dioxide reduction. Nat. Catal. 1, 103–110 (2018).
Tang, W. et al. The importance of surface morphology in controlling the selectivity of polycrystalline copper for CO2 electroreduction. Phys. Chem. Chem. Phys. 14, 76–81 (2012).
Jeon, H. S., Kunze, S., Scholten, F. & Roldan Cuenya, B. Prism-shaped Cu nanocatalysts for electrochemical CO2 reduction to ethylene. ACS Catal. 8, 531–535 (2018).
Reller, C. et al. Selective electroreduction of CO2 toward ethylene on nano dendritic copper catalysts at high current density. Adv. Energy Mater. 7, 1602114 (2017).
Dutta, A., Rahaman, M., Luedi, N. C., Mohos, M. & Broekmann, P. Morphology matters: tuning the product distribution of CO2 electroreduction on oxide-derived Cu foam catalysts. ACS Catal. 6, 3804–3814 (2016).
Huo, Y., Peng, X., Liu, X., Li, H. & Luo, J. High selectivity toward C2H4 production over Cu particles supported by butterfly-wing-derived carbon frameworks. ACS Appl. Mater. Interfaces 10, 12618–12625 (2018).
Hoang, T. T. H. et al. Nanoporous copper–silver alloys by additive-controlled electrodeposition for the selective electroreduction of CO2 to ethylene and ethanol. J. Am. Chem. Soc. 140, 5791–5797 (2018).
Higgins, D., Hahn, C., Xiang, C., Jaramillo, T. F. & Weber, A. Z. Gas-diffusion electrodes for carbon dioxide reduction: a new paradigm. ACS Energy Lett. 4, 317–324 (2019).
Dinh, C.-T. et al. CO2 electroreduction to ethylene via hydroxide-mediated copper catalysis at an abrupt interface. Science 360, 783–787 (2018).
Huan, T. N. et al. Low-cost high-efficiency system for solar-driven conversion of CO2 to hydrocarbons. Proc. Natl Acad. Sci. USA 116, 9735–9740 (2019).
Li, C. W. & Kanan, M. W. CO2 reduction at low overpotential on Cu electrodes resulting from the reduction of thick Cu2O films. J. Am. Chem. Soc. 134, 7231–7234 (2012).
Mistry, H. et al. Highly selective plasma-activated copper catalysts for carbon dioxide reduction to ethylene. Nat. Commun. 7, 12123 (2016).
Checco, A., Hofmann, T., DiMasi, E., Black, C. T. & Ocko, B. M. Morphology of air nanobubbles trapped at hydrophobic nanopatterned surfaces. Nano Lett. 10, 1354–1358 (2010).
Melnichenko, Y. B. et al. Cavitation on deterministically nanostructured surfaces in contact with an aqueous phase: a small-angle neutron scattering study. Langmuir 30, 9985–9990 (2014).
Zheng, D. et al. Salvinia-effect-inspired ‘sticky’ superhydrophobic surfaces by meniscus-confined electrodeposition. Langmuir 33, 13640–13648 (2017).
Kopljar, D., Inan, A., Vindayer, P., Wagner, N. & Klemm, E. Electrochemical reduction of CO2 to formate at high current density using gas diffusion electrodes. J. Appl. Electrochem. 44, 1107–1116 (2014).
Neumann, D. & Woermann, D. Stability of the volume of air trapped on the abdomen of the water spider Argyroneta aquatica. SpringerPlus 2, 694 (2013).
Hokmabad, B. V. & Ghaemi, S. Effect of flow and particle–plastron collision on the longevity of superhydrophobicity. Sci. Rep. 7, 41448 (2017).
Huan, T. N. et al. Porous dendritic copper: an electrocatalyst for highly selective CO2 reduction to formate in water/ionic liquid electrolyte. Chem. Sci. 8, 742–747 (2017).
Huan, T. N. et al. A dendritic nanostructured copper oxide electrocatalyst for the oxygen evolution reaction. Angew. Chem. Int. Ed. 56, 4792–4796 (2017).
Wang, Y., Im, J., Soares, J. W., Steeves, D. M. & Whitten, J. E. Thiol adsorption on and reduction of copper oxide particles and surfaces. Langmuir 32, 3848–3857 (2016).
Dilimon, V. S., Denayer, J., Delhalle, J. & Mekhalif, Z. Electrochemical and spectroscopic study of the self-assembling mechanism of normal and chelating alkanethiols on copper. Langmuir 28, 6857–6865 (2012).
Simpson, J. T., Hunter, S. R. & Aytug, T. Superhydrophobic materials and coatings: a review. Rep. Prog. Phys. 78, 086501 (2015).
Schoenfisch, M. H. & Pemberton, J. E. Air stability of alkanethiol self-assembled monolayers on silver and gold surfaces. J. Am. Chem. Soc. 120, 4502–4513 (1998).
Singh, M. R., Kwon, Y., Lum, Y., Ager, J. W. & Bell, A. T. Hydrolysis of electrolyte cations enhances the electrochemical reduction of CO2 over Ag and Cu. J. Am. Chem. Soc. 138, 13006–13012 (2016).
Kortlever, R., Shen, J., Schouten, K. J. P., Calle-Vallejo, F. & Koper, M. T. M. Catalysts and reaction pathways for the electrochemical reduction of carbon dioxide. J. Phys. Chem. Lett. 6, 4073–4082 (2015).
Liu, X. et al. pH effects on the electrochemical reduction of CO2 towards C2 products on stepped copper. Nat. Commun. 10, 32 (2019).
Rahaman, M., Dutta, A., Zanetti, A. & Broekmann, P. Electrochemical reduction of CO2 into multicarbon alcohols on activated Cu mesh catalysts: an identical location (IL) study. ACS Catal. 7, 7946–7956 (2017).
Chen, C. S., Wan, J. H. & Yeo, B. S. Electrochemical reduction of carbon dioxide to ethane using nanostructured Cu2O-derived copper catalyst and palladium(ii) chloride. J. Phys. Chem. C 119, 26875–26882 (2015).
Zahiri, B., Sow, P. K., Kung, C. H. & Mérida, W. Active control over the wettability from superhydrophobic to superhydrophilic by electrochemically altering the oxidation state in a low voltage range. Adv. Mater. Interfaces 4, 1700121 (2017).
Eilert, A. et al. Subsurface oxygen in oxide-derived copper electrocatalysts for carbon dioxide reduction. J. Phys. Chem. Lett. 8, 285–290 (2017).
Seymour, R. S. & Hetz, S. K. The diving bell and the spider: the physical gill of Argyroneta aquatica. J. Exp. Biol. 214, 2175–2181 (2011).
Mo, H. & Raftery, D. Pre-SAT180, a simple and effective method for residual water suppression. J. Magn. Reson. 190, 1–6 (2008).
Acknowledgements
V.M. acknowledges financial support from CNRS-Cellule Energie and Fondation of Collège de France for the acquisition of the GC equipment. D.W. was supported by an Idex PSL grant (ANR-10-IDEX-001-02 PSL), the Fondation du Collège de France and the Marie Curie PRESTIGE Fellowship programme. S.L. was funded by the Corps des Ponts, des Eaux et des Forêts. X-ray diffraction measurements were carried out by G. Rousse at the Collège de France. SEM images were collected by D. Montero at the Institut des Matériaux de Paris and F. Pillier at the Laboratoire Interfaces et Systèmes Electrochimiques. BET measurements were carried by J. Blanchard at the Laboratoire de Réactivité de Surface at Sorbonne Université.
Author information
Authors and Affiliations
Contributions
D.W., M.F. and V.M. conceived the research. D.W. and S.L. performed electrocatalysis and characterization. N.M. carried out TEM measurements. F.O., S.L. and D.W. carried out the infrared experiments. XPS was carried out by D.M. and P.M. All authors the analysed the data. D.W. wrote the manuscript. S.L., M.F. and V.M. added to the discussion and contributed to the preparation of the manuscript. M.F. and V.M. supervised the work.
Corresponding authors
Ethics declarations
Competing interests
Authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–21 and Supplementary Tables 1–6.
Supplementary Video 1
Capture and retention of a gaseous CO2 stream by the hydrophobic Cu dendrite.
Rights and permissions
About this article
Cite this article
Wakerley, D., Lamaison, S., Ozanam, F. et al. Bio-inspired hydrophobicity promotes CO2 reduction on a Cu surface. Nat. Mater. 18, 1222–1227 (2019). https://doi.org/10.1038/s41563-019-0445-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41563-019-0445-x
This article is cited by
-
Ligand-modified nanoparticle surfaces influence CO electroreduction selectivity
Nature Communications (2024)
-
Gas diffusion enhanced electrode with ultrathin superhydrophobic macropore structure for acidic CO2 electroreduction
Nature Communications (2024)
-
Vitamin C-induced CO2 capture enables high-rate ethylene production in CO2 electroreduction
Nature Communications (2024)
-
Extrinsic hydrophobicity-controlled silver nanoparticles as efficient and stable catalysts for CO2 electrolysis
Nature Communications (2024)
-
Advances in bio-inspired electrocatalysts for clean energy future
Nano Research (2024)