Abstract
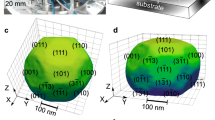
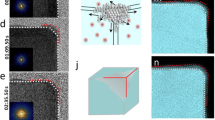
Crystal facets, vertices and edges govern the energy landscape of metal surfaces and thus the chemical interactions on the surface1,2. The facile absorption and desorption of hydrogen at a palladium surface provides a useful platform for defining how metal–solute interactions impact properties relevant to energy storage, catalysis and sensing3,4,5. Recent advances in in operando and in situ techniques have enabled the phase transitions of single palladium nanocrystals to be temporally and spatially tracked during hydrogen absorption6,7,8,9,10,11. We demonstrate herein that in situ X-ray diffraction can be used to track both hydrogen absorption and desorption in palladium nanocrystals. This ensemble measurement enabled us to delineate distinctive absorption and desorption mechanisms for nanocrystals containing exclusively (111) or (100) facets. We show that the rate of hydrogen absorption is higher for those nanocrystals containing a higher number of vertices, consistent with hydrogen absorption occurring quickly after β-phase nucleation at lattice-strained vertices9,10. Tracking hydrogen desorption revealed initial desorption rates to be nearly tenfold faster for samples with (100) facets, presumably due to the faster recombination of surface hydrogen atoms. These results inspired us to make nanocrystals with a high number of vertices and (100) facets, which were found to accommodate fast hydrogen uptake and release.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All the data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Calle-Vallejo, F., Loffreda, D., Koper, M. T. M. & Sautet, P. Introducing structural sensitivity into adsorption—energy scaling relations by means of coordination numbers. Nat. Chem. 7, 403–410 (2015).
Lee, I., Delbecq, F., Morales, R., Albiter, M. A. & Zaera, F. Tuning selectivity in catalysis by controlling particle shape. Nat. Mater. 8, 132–138 (2009).
Teschner, D. et al. The roles of subsurface carbon and hydrogen in palladium-catalyzed alkyne hydrogenation. Science 320, 86–89 (2008).
Li, G. et al. Hydrogen storage in Pd nanocrystals covered with a metal–organic framework. Nat. Mater. 13, 802–806 (2014).
Liu, N., Tang, M. L., Hentschel, M., Giessen, H. & Alivisatos, A. P. Nanoantenna-enhanced gas sensing in a single tailored nanofocus. Nat. Mater. 10, 631–636 (2011).
Bardhan, R. et al. Uncovering the intrinsic size dependence of hydriding phase transformations in nanocrystals. Nat. Mater. 12, 905–912 (2013).
Syrenova, S. et al. Hydride formation thermodynamics and hysteresis in individual Pd nanocrystals with different size and shape. Nat. Mater. 14, 1236–1244 (2015).
Griessen, R., Strohfeldt, N. & Giessen, H. Thermodynamics of the hybrid interaction of hydrogen with palladium nanoparticles. Nat. Mater. 15, 311–317 (2016).
Ulvestad, A. et al. Avalanching strain dynamics during the hydriding phase transformation in individual palladium nanoparticles. Nat. Commun. 6, 10092 (2015).
Narayan, T. C. et al. Direct visualization of hydrogen absorption dynamics in individual palladium nanoparticles. Nat. Commun. 8, 14020 (2017).
Narayan, T. C., Baldi, A., Koh, A. L., Sinclair, R. & Dionne, J. A. Reconstructing solute-induced phase transformations within individual nanocrystals. Nat. Mater. 15, 768–774 (2016).
Wicke, E, Brodowsky, H. & Züchner, H. in Hydrogen in Metals II 73–155 (Springer, 1978).
Baldi, A., Narayan, T. C., Koh, A. L. & Dionne, J. A. In situ detection of hydrogen-induced phase transitions in individual palladium nanocrystals. Nat. Mater. 13, 1143–1148 (2014).
Ulvestad, A. et al. Three-dimensional imaging of dislocation dynamics during the hydriding phase transformation. Nat. Mater. 16, 565–571 (2017).
Klinkova, A. et al. Shape-dependent interactions of palladium nanocrystals with hydrogen. Small 12, 2450–2458 (2016).
Lim, B. et al. Shape-controlled synthesis of Pd nanocrystals in aqueous solutions. Adv. Funct. Mater. 19, 189–200 (2009).
Sytwu, K. et al. Visualizing facet-dependent hydrogenation dynamics in individual palladium nanoparticles. Nano Lett. 9, 5357–5363 (2018).
Li, G. et al. Shape-dependent hydrogen-storage properties in Pd nanocrystals: which does hydrogen prefer, octahedron (111) or cube (100)? J. Am. Chem. Soc. 136, 10222–10225 (2014).
Zalineeva, A., Baranton, S., Coutanceau, C. & Jerkiewicz, G. Octahedral palladium nanoparticles as excellent hosts for electrochemically adsorbed and absorbed hydrogen. Sci. Adv. 3, e1600542 (2017).
Sherbo, R. S. et al. Accurate coulometric quantification of hydrogen absorption in palladium nanoparticles and thin films. Chem. Mater. 30, 3963–3970 (2018).
Satterfield, C. N. Heterogeneous Catalysis in Practice (McGraw-Hill, 1980).
Stern, A., Resnik, A. & Shaltiel, D. Thermal desorption spectra of the PdHx system in a powder form. J. Phys. F 14, 1625–1639 (1984).
Savara, A., Ludwig, W. & Schauermann, S. Kinetic evidence for a non-Langmuir–Hinshelwood surface reaction: H/D exchange over Pd nanoparticles and Pd(111). ChemPhysChem 14, 1686–1695 (2013).
Jewell, L. L. & Davis, B. H. Review of absorption and adsorption in the hydrogen–palladium system. Appl. Catal. A 310, 1–15 (2006).
Shen, X. et al. Hydrogen diffusion into the subsurfaces of model metal catalysts from first principles. Phys. Chem. Chem. Phys. 19, 3557–3564 (2017).
Kobayashi, H. et al. On the nature of strong hydrogen atom trapping inside Pd nanoparticles. J. Am. Chem. Soc. 130, 1828–1829 (2008).
Crespo-Quesada, M. et al. UV–ozone cleaning of supported poly(vinylpyrrolidone)-stabilized palladium nanocubes: effect of stabilizer removal on morphology and catalytic behavior. Langmuir 27, 7909–7916 (2011).
Aricò, A. S., Bruce, P., Scrosati, B., Tarascon, J.-M. & van Schalkwijk, W. Nanostructured materials for advanced energy conversion and storage devices. Nat. Mater. 4, 366–377 (2005).
Huiberts, J. N. et al. Yttrium and lanthanum hydride films with switchable optical properties. Nature 380, 231–234 (1996).
Sherbo, R. S., Delima, R. S., Chiykowski, V. A., MacLeod, B. P. & Berlinguette, C. P. Complete electron economy by pairing electrolysis with hydrogenation. Nat. Catal. 1, 501–507 (2018).
Acknowledgements
The authors acknowledge financial support from the Canadian Natural Science and Engineering Council (RGPIN 337345-13), Canadian Foundation for Innovation (229288), Canadian Institute for Advanced Research (BSE-BERL-162173), Collaborative Research and Development Grant (CRD-502052-16), Canada Research Chairs and Google LLC. This work made use of the 4D LABS shared facilities supported by the Canada Foundation for Innovation (CFI), British Columbia Knowledge Development Fund (BCKDF), Western Economic Diversification Canada (WD) and Simon Fraser University (SFU).
Author information
Authors and Affiliations
Contributions
N.J.J.J., B.L. and C.P.B. devised the concept. N.J.J.J. synthesized and characterized the nanocrystals. B.L. designed and performed in situ studies. B.P.M. performed statistical analysis of experimental data. R.S.S. and M.M.-G. performed electrochemical hydrogen loading experiments. D.K.F. provided experimental and technical advice. N.J.J.J., B.L. and C.P.B. wrote the manuscript with input from all authors. C.P.B. supervised the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary Information
Supplementary Figures 1–16, Supplementary Tables 1,2, Supplementary Discussion
Rights and permissions
About this article
Cite this article
Johnson, N.J.J., Lam, B., MacLeod, B.P. et al. Facets and vertices regulate hydrogen uptake and release in palladium nanocrystals. Nat. Mater. 18, 454–458 (2019). https://doi.org/10.1038/s41563-019-0308-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41563-019-0308-5
This article is cited by
-
Impact of palladium/palladium hydride conversion on electrochemical CO2 reduction via in-situ transmission electron microscopy and diffraction
Nature Communications (2024)
-
Naked-eye observation of water-forming reaction on palladium etalon: transduction of gas-matter reaction into light-matter interaction
PhotoniX (2023)
-
Preparation and hydrogen detection performance of 3D self-assembled Pd nanoflowers
Journal of Materials Science: Materials in Electronics (2022)
-
Design concept for electrocatalysts
Nano Research (2022)
-
Synthesis of PdH0.43 nanocrystals with different surface structures and their catalytic activities towards formic acid electro-oxidation
Science China Materials (2020)