Abstract
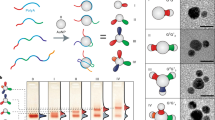
Surface encoding of colloidal nanoparticles with DNA is fundamental for fields where recognition interaction is required, particularly controllable material self-assembly. However, regioselective surface encoding of nanoparticles is still challenging because of the difficulty associated with breaking the identical chemical environment on nanoparticle surfaces. Here we demonstrate the selective blocking of nanoparticle surfaces with a diblock copolymer (polystyrene-b-polyacrylic acid). By tuning the interfacial free energies of a ternary system involving the nanoparticles, solvent and copolymer, controllable accessibilities to the nanoparticles’ surfaces are obtained. Through the modification of the polymer-free surface region with single-stranded DNA, regioselective and programmable surface encoding is realized. The resultant interparticle binding potential is selective and directional, allowing for an increased degree of complexity of potential self-assemblies. The versatility of this regioselective surface encoding strategy is demonstrated on various nanoparticles of isotropic or anisotropic shape and a total of 24 distinct complex nanoassemblies are fabricated.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Change history
12 December 2018
In the version of this Article originally published, the diblock copolymer structure in Fig. 2a showed a single bond between the carbon and the oxygen atoms; it should have been a double bond. This has been corrected in all versions of the Article.
References
Xia, Y., Xiong, Y., Lim, B. & Skrabalak, S. E. Shape-controlled synthesis of metal nanocrystals: simple chemistry meets complex physics? Angew. Chem. Int. Ed. 48, 60–103 (2009).
Huang, X., Neretina, S. & El-Sayed, M. A. Gold nanorods: from synthesis and properties to biological and biomedical applications. Adv. Mater. 21, 4880–4910 (2009).
Prodan, E., Radloff, C., Halas, N. J. & Nordlander, P. A hybridization model for the plasmon response of complex nanostructures. Science 302, 419–422 (2003).
Sun, Y. G. & Xia, Y. N. Shape-controlled synthesis of gold and silver nanoparticles. Science 298, 2176–2179 (2002).
Xia, Y. N. et al. One-dimensional nanostructures: Synthesis, characterization, and applications. Adv. Mater. 15, 353–389 (2003).
Langille, M. R., Zhang, J., Personick, M. L., Li, S. & Mirkin, C. A. Stepwise evolution of spherical seeds into 20-fold twinned icosahedra. Science 337, 954–957 (2012).
Jin, R. C. et al. Controlling anisotropic nanoparticle growth through plasmon excitation. Nature 425, 487–490 (2003).
Zhu, M., Aikens, C. M., Hollander, F. J., Schatz, G. C. & Jin, R. Correlating the crystal structure of a thiol-protected Au-25 cluster and optical properties. J. Am. Chem. Soc. 130, 5883–5884 (2008).
Alivisatos, A. P. Semiconductor clusters, nanocrystals, and quantum dots. Science 271, 933–937 (1996).
Sun, S. H. & Zeng, H. Size-controlled synthesis of magnetite nanoparticles. J. Am. Chem. Soc. 124, 8204–8205 (2002).
Lee, J.-H., Gibson, K. J., Chen, G. & Weizmann, Y. Bipyramid-templated synthesis of monodisperse anisotropic gold nanocrystals. Nat. Commun. 6, 7571 (2015).
Nie, Z., Petukhova, A. & Kumacheva, E. Properties and emerging applications of self-assembled structures made from inorganic nanoparticles. Nat. Nanotech. 5, 15–25 (2010).
Li, F., Josephson, D. P. & Stein, A. Colloidal assembly: the road from particles to colloidal molecules and crystals. Angew. Chem. Int. Ed. 50, 360–388 (2011).
Kuzyk, A. et al. DNA-based self-assembly of chiral plasmonic nanostructures with tailored optical response. Nature 483, 311–314 (2012).
Park, S. Y. et al. DNA-programmable nanoparticle crystallization. Nature 451, 553–556 (2008).
Shevchenko, E. V., Talapin, D. V., Kotov, N. A., O’Brien, S. & Murray, C. B. Structural diversity in binary nanoparticle superlattices. Nature 439, 55–59 (2006).
Whitesides, G. M. & Grzybowski, B. Self-assembly at all scales. Science 295, 2418–2421 (2002).
Nykypanchuk, D., Maye, M. M., van der Lelie, D. & Gang, O. DNA-guided crystallization of colloidal nanoparticles. Nature 451, 549–552 (2008).
Singh, G. et al. Self-assembly of magnetite nanocubes into helical superstructures. Science 345, 1149–1153 (2014).
Alivisatos, A. P. et al. Organization of ‘nanocrystal molecules’ using DNA. Nature 382, 609–611 (1996).
Edwardson, T. G. W., Lau, K. L., Bousmail, D., Serpell, C. J. & Sleiman, H. F. Transfer of molecular recognition information from DNA nanostructures to gold nanoparticles. Nat. Chem. 8, 162–170 (2016).
Xu, X., Rosi, N. L., Wang, Y., Huo, F. & Mirkin, C. A. Asymmetric functionalization of gold nanoparticles with oligonucleotides. J. Am. Chem. Soc. 128, 9286–9287 (2006).
Liu, W., Halverson, J., Tian, Y., Tkachenko, A. V. & Gang, O. Self-organized architectures from assorted DNA-framed nanoparticles. Nat. Chem. 8, 867–873 (2016).
Liu, W. et al. Diamond family of nanoparticle superlattices. Science 351, 582–586 (2016).
Shen, C. et al. Site-specific surface functionalization of gold nanorods using DNA origami clamps. J. Am. Chem. Soc. 138, 1764–1767 (2016).
Maye, M. M., Nykypanchuk, D., Cuisinier, M., van der Lelie, D. & Gang, O. Stepwise surface encoding for high-throughput assembly of nanoclusters. Nat. Mater. 8, 388–391 (2009).
Pan, Y., Gao, J. H., Zhang, B., Zhang, X. X. & Xu, B. Colloidosome-based synthesis of a multifunctional nanostructure of silver and hollow iron oxide nanoparticles. Langmuir 26, 4184–4187 (2010).
Walther, A. & Mueller, A. H. E. Janus particles: synthesis, self-assembly, physical properties, and applications. Chem. Rev. 113, 5194–5261 (2013).
Chen, Q. et al. Supracolloidal reaction kinetics of Janus spheres. Science 331, 199–202 (2011).
Xing, H. et al. DNA-directed assembly of asymmetric nanoclusters using Janus nanoparticles. ACS Nano 6, 802–809 (2012).
Wang, F., Cheng, S., Bao, Z. & Wang, J. Anisotropic overgrowth of metal heterostructures induced by a site-selective silica coating. Angew. Chem. Int. Ed. 52, 10344–10348 (2013).
Chen, T. et al. Hotspot-induced transformation of surface-enhanced Raman scattering fingerprints. ACS Nano 4, 3087–3094 (2010).
Li, Y. L., Liu, Z. Y., Yu, G. M., Jiang, W. & Mao, C. D. Self-assembly of molecule-like nanoparticle clusters directed by DNA nanocages. J. Am. Chem. Soc. 137, 4320–4323 (2015).
Liu, K. et al. Step-growth polymerization of inorganic nanoparticles. Science 329, 197–200 (2010).
Walker, D. A., Leitsch, E. K., Nap, R. J., Szleifer, I. & Grzybowski, B. A. Geometric curvature controls the chemical patchiness and self-assembly of nanoparticles. Nat. Nanotech. 8, 676–681 (2013).
Choueiri, R. M. et al. Surface patterning of nanoparticles with polymer patches. Nature 538, 79–83 (2016).
Chen, T., Yang, M., Wang, X., Tan, L. H. & Chen, H. Controlled assembly of eccentrically encapsulated gold nanoparticles. J. Am. Chem. Soc. 130, 11858–11859 (2008).
Torza, S. & Mason, S. G. Three-phase interactions in shear and electrical fields. J. Colloid. Interface. Sci. 33, 67–83 (1970).
Boettcher, M. & McManus, M. T. Choosing the right tool for the job: RNAi, TALEN, or CRISPR. Mol. Cell 58, 575–585 (2015).
Love, J. C., Estroff, L. A., Kriebel, J. K., Nuzzo, R. G. & Whitesides, G. M. Self-assembled monolayers of thiolates on metals as a form of nanotechnology. Chem. Rev. 105, 1103–1170 (2005).
Zhang, L. & Eisenberg, A. Morphogenic effect of added ions on crew-cut aggregates of polystyrene-b-poly(acrylic acid) block copolymers in solutions. Macromolecules 29, 8805–8815 (1996).
Hurst, S. J., Lytton-Jean, A. K. R. & Mirkin, C. A. Maximizing DNA loading on a range of gold nanoparticle sizes. Anal. Chem. 78, 8313–8318 (2006).
Yang, M. et al. Mechanistic investigation into the spontaneous linear assembly of gold nanospheres. Phys. Chem. Chem. Phys. 12, 11850–11860 (2010).
Nikoobakht, B. & El-Sayed, M. A. Preparation and growth mechanism of gold nanorods (NRs) using seed-mediated growth method. Chem. Mater. 15, 1957–1962 (2003).
Orendorff, C. J. & Murphy, C. J. Quantitation of metal content in the silver-assisted growth of gold nanorods. J. Phys. Chem. B 110, 3990–3994 (2006).
Scarabelli, L., Coronado-Puchau, M., Giner-Casares, J. J., Langer, J. & Liz-Marzan, L. M. Monodisperse gold nanotriangles: size control, large-scale self-assembly, and performance in surface-enhanced Raman scattering. ACS Nano 8, 5833–5842 (2014).
Kou, X., Sun, Z., Yang, Z., Chen, H. & Wang, J. Curvature-directed assembly of gold nanocubes, nanobranches, and nanospheres. Langmuir 25, 1692–1698 (2009).
Park, K., Koerner, H. & Vaia, R. A. Depletion-induced shape and size selection of gold nanoparticles. Nano. Lett. 10, 1433–1439 (2010).
Chen, H. et al. Plasmon coupling in clusters composed of two-dimensionally ordered gold nanocubes. Small 5, 2111–2119 (2009).
Asakura, S. & Oosawa, F. On interaction between two bodies immersed in a solution of macromolecules. J. Chem. Phys. 22, 1255–1256 (1954).
Berr, S. S. Solvent isotope effects on alkyltrimethylammonium bromide micelles as a function of alkyl chain-length. J. Phys. Chem. 91, 4760–4765 (1987).
Bakshi, M. S. & Kaur, I. Head-group-induced structural micellar transitions in mixed cationic surfactants with identical hydrophobic tails. Colloid Polym. Sci. 281, 10–18 (2003).
Acknowledgements
This work is supported by the University of Chicago and the NSF CAREER Award (DMR-1555361) to Y.W. D.L. acknowledges the Martha Ann and Joseph A. Chenicek Graduate Research Fund and HHMI International Student Research Fellowship. This research used resources of the Center for Functional Nanomaterials at Brookhaven National Laboratory, which is supported by US DOE Office of Science Facilities under Contract DE-SC0012704. O.G. gratefully acknowledges the support by the US Department of Energy, Office of Basic Energy Sciences, Division of Materials Sciences and Engineering under grant no. DE-SC0008772.
Author information
Authors and Affiliations
Contributions
G.C. and Y.W. conceived the idea. G.C. and D.L. designed the experiments and developed the methodology. During the revision, H.C.R. and K.J.G. helped with the NP synthesis, K.J.G. performed the polymer encapsulation and self-assemblies, J.-H.L. helped with the microscopy. W.X., R.L. and H.L.X. performed the microscopy for the 3D tomograms. H.L.X. and O.G. analysed the data for the 3D reconstructions. Y.W. supervised the project. G.C., D.L., K.J.G., H.C.R. and Y.W. analysed the data and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Video Legends 1–6, Supplementary Figures 1–37, Supplementary Tables 1 and 2 and Supplementary References
Supplementary Video 1
3D tomographic reconstruction of v-AuNC partially encapsulated nanostructure
Supplementary Video 2
Full rotation of 3D tomographic reconstruction of c-AuNC self-assembly with AuNS
Supplementary Video 3
Full rotation of 3D tomographic reconstruction of c-AuNC partially encapsulated nanostructure
Supplementary Video 4
Half rotation at slower speed of 3D tomographic reconstruction of c-AuNC partially encapsulated nanostructure
Supplementary Video 5
3D tomographic reconstruction showing detailed surface structure of the c-AuNC through a sliding partition plane
Supplementary Video 6
Full rotation of 3D tomographic reconstruction of c-AuNC self-assembly with AuNS
Rights and permissions
About this article
Cite this article
Chen, G., Gibson, K.J., Liu, D. et al. Regioselective surface encoding of nanoparticles for programmable self-assembly. Nature Mater 18, 169–174 (2019). https://doi.org/10.1038/s41563-018-0231-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41563-018-0231-1
This article is cited by
-
No ground truth needed: unsupervised sinogram inpainting for nanoparticle electron tomography (UsiNet) to correct missing wedges
npj Computational Materials (2024)
-
Synthesis of branched silica nanotrees using a nanodroplet sequential fusion strategy
Nature Synthesis (2023)
-
Templated synthesis of patterned gold nanoparticle assemblies for highly sensitive and reliable SERS substrates
Nano Research (2023)
-
Symmetry-breaking in patch formation on triangular gold nanoparticles by asymmetric polymer grafting
Nature Communications (2022)
-
Self-regulated co-assembly of soft and hard nanoparticles
Nature Communications (2021)