Abstract
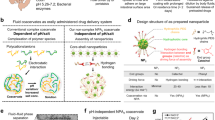
The gastrointestinal tract is the site of most drug delivery and therapeutic interventions for the management and treatment of numerous diseases. However, selective access to its mucosa, especially in the small bowel, is challenging. Here we develop an orally administered gut-coating formulation that provides a transient coating of the bowel. Through a materials screening campaign, we identified a sucrose octasulfate aluminium complex and further engineered the pH-dependent material into a complex coacervate formulation linked via pH-independent electrostatic interaction, which allowed an effective transient physical coating on the gastrointestinal mucosa, independent of gastric acid exposure. We tested the therapeutic values of this technology in two settings. Oral administration of this gut-coating formulation modulated the nutrient contact with bowel mucosa, which lowered the glucose responses in rodent models indicating a potential therapeutic utility in diabetes. Furthermore, the formulation protected biological agents from gastric acid exposure and degradation, which enabled oral delivery to the small bowel mucosa.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
American Diabetes Association 2 Classification and diagnosis of diabetes. Diabetes Care 39 S13–S22; erratum. Diabetes Care 39, 1653 (2016).
Mingrone, G. et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N. Engl. J. Med 366, 1577–1585 (2012).
Lovshin, J. A. & Drucker, D. J. Incretin-based therapies for type 2 diabetes mellitus.Nat. Rev. Endocrinol. 5, 262–269 (2009).
Drucker, D. J. & Nauck, M. A. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 368, 1696–1705 (2006).
Stefater, M. A., Wilson-Pérez, H. E., Chambers, A. P., Sandoval, D. A. & Seeley, R. J. All bariatric surgeries are not created equal: insights from mechanistic comparisons. Endocr. Rev. 33, 595–622 (2012).
Rubino, F. et al. The early effect of the Roux-en-Y gastric bypass on hormones involved in body weight regulation and glucose metabolism. Ann. Surg. 240, 236–242 (2004).
Jorgensen, N. B. et al. Acute and long-term effects of Roux-en-Y gastric bypass on glucose metabolism in subjects with Type 2 diabetes and normal glucose tolerance. Am. J. Physiol. Endocrinol. Metab. 303, E122–E131 (2012).
Zhang, S., Bellinger, A. M., Glettig, D. L. & Barman, R. A pH-responsive supramolecular polymer gel as an enteric elastomer for use in gastric devices. Nat. Mater. 14, 1065–1073 (2015).
Zelikin, A. N., Ehrhardt, C. & Healy, A. M. Materials and methods for delivery of biological drugs. Nat. Chem. 8, 997–1007 (2016).
Fuhrmann, G. Sustained gastrointestinal activity of dendronized polymer–enzyme conjugates. Nat. Chem. 5, 582–589 (2013).
Danesh, B. J., Duncan, A. & Russell, R. I. Is an acid pH medium required for the protective effect of sucralfate against mucosal injury? Am. J. Med. 83, 11–13 (1987).
Morris, G. P. (eds D. Hollander & G. Tytgat) Sucralfate: From Basic Science to the Bedside. 71–82 (Springer, New York, NY, 1995).
Nagashima, R. Mechanisms of action of sucralfate. J. Clin. Gastroenterol. 3, 117–127 (1981).
Ochi, K. (eds D. Hollander & G. Tytgat) Sucralfate: From Basic Science to the Bedside. 47–58 (Springer, New York, NY, 1995).
McCarthy, D. M. Drug therapy: sucralfate. New Engl. J. Med. 325, 1017–1025 (1991).
Nail, S. L., White, J. L. & Hem, S. L. Structure of aluminum hydroxide gel. I: Initial precipitate. J. Pharm. Sci. 65, 1188–1191 (1976).
Hem, J. D. & Roberson, C. E. Form and Stability of Aluminum Hydroxide Complexes in Dilute Solution Water Supply Paper 1827-A (Geological Survey, Washington DC, 1967).
Wang, Q. & Schlenoff, J. B. The polyelectrolyte complex/coacervate continuum. Macromolecules 47, 3108–3116 (2014).
de Kruif, C. G., Weinbreck, F. & de Vries, R. Complex coacervation of proteins and anionic polysaccharides. Curr. Opin. Colloid Interface Sci. 9, 340–349 (2004).
Veis, A. & Aranyi, C. Phase separation in polyelectrolyte systems. I. Complex coacervates of gelatin. J. Phys. Chem. 64, 1203–1210 (1960).
Cummings, D. E. & Flum, D. R. Gastrointestinal surgery as a treatment for diabetes. J. Am. Med. Assoc. 299, 341–343 (2008).
Couzin, J. Bypassing medicine to treat diabetes. Science 320, 438–440 (2008).
Betzel, B. et al. Weight reduction and improvement in diabetes by the duodenal–jejunal bypass liner: a 198 patient cohort study. Surg. Endosc. 31, 2881–2891 (2016).
Koehestanie, P. et al. Duodenal–jejunal bypass liner implantation provokes rapid weight loss and improved glycemic control, accompanied by elevated fasting ghrelin levels. Endosc. Int. Open 2, E21–E27 (2014).
Cohen, R. et al. Role of proximal gut exclusion from food on glucose homeostasis in patients with Type 2 diabetes. Diabetes Med. 30, 1482–1486 (2013).
Acknowledgements
This work was supported by NIH grant GM086433 to J.M.K., NIH grant DK084064 to A.T., Partners Innovation Development Grants Program and BRI Translational Technologies and Care Innovation Grant from Brigham Research Institute (BRI) to J.M.K. and A.T., Diabetes Action Research and Education Foundation Grant to J.M.K., Accelerator Award from CIMIT to J.M.K. and A.T., the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education of Korea (2012R1A6A3A03041166) and the Korea Institute for Advancement of Technology (N0002123) to Y.L. This work was supported in part by the Netherland–America Foundation (NAF) Fulbright Fellowship, the Ivy Circle Award and the Prince Bernard Culture Foundation Award to T.E.D. We thank J.N.M. IJzermans at Erasmus University Medical Center, Rotterdam, for his role as educational supervisor to T.E.D. The authors thank J. Tolkoff and F. Schoen for critical feedback. We thank S. Wang for the CT imaging and W. Li for the IVIS imaging.
Author information
Authors and Affiliations
Contributions
Y.L., T.E.D., A.T. and J.M.K. developed the concept and designed experiments. Y.L., T.E.D., K.C. and D.S.Y.L conducted the experiments. Y.L., T.E.D., K.C., D.S.Y.L., A.T. and J.M.K. analysed the data. Y.L., T.E.D., A.T. and J.M.K. wrote the manuscript. All the authors provided critical comments on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods, Supplementary Figures 1–10, Supplementary Table 1, Supplementary Movie Captions 1–7, Supplementary References 1–6
Supplementary Movie 1
Representative 3D projection CT image of SD rats gavaged with 450 mg kg–1 rat sucralfate (dosage calculated based on the rat weight) 1 hr before the CT imaging
Supplementary Movie 2
Representative 3D projection CT image of SD rats gavaged with 450 mg kg–1 rat sucralfate (dosage calculated based on the rat weight) 2.5 hr before the CT imaging
Supplementary Movie 3
Video clip showing LuCI paste hydrated in SIF (pH 6.5) manually spread onto a freshly harvested rat intestine mucosa
Supplementary Movie 4
Video clip showing LuCI paste hydrated in SIF (pH 6.5) manually spread onto a freshly harvested rat intestine mucosa (the same sample from Movie S3) and shaken in normal saline
Supplementary Movie 5
Representative 3D projection CT image of SD rats gavaged with 450 mg kg–1 rat LuCI (dosage calculated based on the rat weight) 1 hr before the CT imaging
Supplementary Movie 6
Representative 3D projection CT image of SD rats gavaged with 450 mg kg–1 rat LuCI (dosage calculated based on the rat weight) 5 hr before the CT imaging
Supplementary Movie 7
Representative 3D projection CT image of SD rats gavaged with 450 mg kg–1 rat LuCI (dosage calculated based on the rat weight) 24 hr before the CT imaging
Rights and permissions
About this article
Cite this article
Lee, Y., Deelman, T.E., Chen, K. et al. Therapeutic luminal coating of the intestine. Nature Mater 17, 834–842 (2018). https://doi.org/10.1038/s41563-018-0106-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41563-018-0106-5
This article is cited by
-
A multifunctional integrated biomimetic spore nanoplatform for successively overcoming oral biological barriers
Journal of Nanobiotechnology (2023)
-
A soil-inspired dynamically responsive chemical system for microbial modulation
Nature Chemistry (2023)
-
A single-cell nanocoating of probiotics for enhanced amelioration of antibiotic-associated diarrhea
Nature Communications (2022)
-
Nanoparticle-assembled bioadhesive coacervate coating with prolonged gastrointestinal retention for inflammatory bowel disease therapy
Nature Communications (2021)
-
An orally delivered microbial cocktail for the removal of nitrogenous metabolic waste in animal models of kidney failure
Nature Biomedical Engineering (2020)