Abstract
Rising atmospheric CO2 has stimulated plant productivity, with terrestrial ecosystems currently absorbing nearly one-third of anthropogenic CO2 emissions. Increases in photosynthesis can subsequently lead to increased carbon (C) storage in plants and soil. However, there is growing evidence that nitrogen (N) availability constrains elevated CO2 (eCO2) responses, yet we know much less about the role of phosphorus (P) limitation on productivity under eCO2. This is important because P-limited ecosystems are globally widespread, and the biogeochemical cycles of N and P differ fundamentally. In the Peak District National Park of northern England, we conducted a free-air CO2 enrichment (FACE) experiment for three years on two contrasting P-limited grasslands under long-term nutrient manipulation. Here we show that competition between plants and microbes for P can determine plant productivity responses to eCO2. In a limestone grassland, aboveground productivity increased (16%) and microbial biomass P remained unchanged, whereas in an acidic grassland, aboveground productivity and P uptake declined (11% and 20%, respectively), but P immobilization into microbial biomass increased (36%). Our results demonstrate that strong competition with microbes can cause plant P uptake to decline under eCO2, with implications for the future productivity of P-limited ecosystems in response to climate change.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
Data used to produce this paper are available via the EIDC data repository https://doi.org/10.5285/35921c93-2d9e-4e35-8de5-adbfc37641b4.
References
IPCC Climate Change 2021: The Physical Science Basis (eds Masson-Delmotte et al.) (Cambridge Univ. Press, 2021).
Reich, P. B. et al. Nitrogen limitation constrains sustainability of ecosystem response to CO2. Nature 440, 922–925 (2006).
Zavalloni, C. et al. Exposure to warming and CO2 enrichment promotes greater above-ground biomass, nitrogen, phosphorus and arbuscular mycorrhizal colonization in newly established grasslands. Plant Soil 359, 121–136 (2012).
Reich, P. B. & Hobbie, S. E. Decade-long soil nitrogen constraint on the CO2 fertilization of plant biomass. Nat. Clim. Change 3, 278–282 (2013).
Zaehle, S., Jones, C. D., Houlton, B., Lamarque, J. F. & Robertson, E. Nitrogen availability reduces CMIP5 projections of twenty-first-century land carbon uptake. J. Clim. 28, 2494–2511 (2015).
Goll, D. S. et al. Nutrient limitation reduces land carbon uptake in simulations with a model of combined carbon, nitrogen and phosphorus cycling. Biogeosciences 9, 3547–3569 (2012).
Du, E. Z. et al. Global patterns of terrestrial nitrogen and phosphorus limitation. Nat. Geosci. 13, 221–226 (2020).
Zhang, Q., Wang, Y. P., Matear, R. J., Pitman, A. J. & Dai, Y. J. Nitrogen and phosphorous limitations significantly reduce future allowable CO2 emissions. Geophys. Res. Lett. 41, 632–637 (2014).
Ellsworth, D. S. et al. Elevated CO2 does not increase eucalypt forest productivity on a low phosphorus soil. Nat. Clim. Change 7, 279–282 (2017).
Horswill, P., O’Sullivan, O., Phoenix, G. K., Lee, J. A. & Leake, J. R. Base cation depletion, eutrophication and acidification of species-rich grasslands in response to long-term simulated nitrogen deposition. Environ. Pollut. 155, 336–349 (2008).
Turner, B. L. et al. Soil microbial biomass and the fate of phosphorus during long-term ecosystem development. Plant Soil 367, 225–234 (2013).
Vance, C. P., Uhde-Stone, C. & Allan, D. L. Phosphorus acquisition and use: critical adaptations by plants for securing a non-renewable resource. N. Phytol. 157, 423–447 (2003).
Keane, J. B. et al. Soil C, N and P cycling enzyme responses to nutrient limitation under elevated CO2. Biogeochemistry 151, 221–235 (2020).
Terrer, C., Vicca, S., Hungate, B. A., Phillips, R. P. & Prentice, I. C. Mycorrhizal association as a primary control of the CO2 fertilization effect. Science 353, 72–74 (2016).
Drake, J. E. et al. Short-term carbon cycling responses of a mature eucalypt woodland to gradual stepwise enrichment of atmospheric CO2 concentration. Glob. Change Biol. 22, 380–390 (2016).
Jiang, M. K. et al. The fate of carbon in a mature forest under carbon dioxide enrichment. Nature 580, 227–231 (2020).
Terrer, C. et al. Nitrogen and phosphorus constrain the CO2 fertilization of global plant biomass. Nat. Clim. Change 9, 684–689 (2019).
Jiang, M. K. et al. Low phosphorus supply constrains plant responses to elevated CO2: a meta-analysis. Glob. Change Biol. 26, 5856–5873 (2020).
Chapin, F. S., Matson, P. A., Mooney, H. A. & Vitousek, P. M. Principles of Terrestrial Ecosystem Ecology (Springer Verlag, 2002).
Phoenix, G. K. et al. Effects of enhanced nitrogen deposition and phosphorus limitation on nitrogen budgets of semi-natural grasslands. Glob. Change Biol. 9, 1309–1321 (2003).
Bunemann, E. K. et al. Rapid microbial phosphorus immobilization dominates gross phosphorus fluxes in a grassland soil with low inorganic phosphorus availability. Soil Biol. Biochem. 51, 84–95 (2012).
Chen, J. et al. Long-term nitrogen loading alleviates phosphorus limitation in terrestrial ecosystems. Glob. Change Biol. 26, 5077–5086 (2020).
Hebeisen, T. et al. Growth response of Trifolium repens L and Lolium perenne L as monocultures and bi-species mixture to free air CO2 enrichment and management. Glob. Change Biol. 3, 149–160 (1997).
Kammann, C., Grunhage, L., Gruters, U., Janze, S. & Jager, H. J. Response of aboveground grassland biomass and soil moisture to moderate long-term CO2 enrichment. Basic Appl. Ecol. 6, 351–365 (2005).
Jonasson, S., Michelsen, A. & Schmidt, I. K. Coupling of nutrient cycling and carbon dynamics in the Arctic, integration of soil microbial and plant processes. Appl. Soil Ecol. 11, 135–146 (1999).
Hodge, A., Robinson, D. & Fitter, A. Are microorganisms more effective than plants at competing for nitrogen? Trends Plant Sci. 5, 304–308 (2000).
Liu, Q. Y. et al. Nitrogen acquisition by plants and microorganisms in a temperate grassland. Sci. Rep. 6, 22642 (2016).
Dijkstra, F. A., He, M. Z., Johansen, M. P., Harrison, J. J. & Keitel, C. Plant and microbial uptake of nitrogen and phosphorus affected by drought using N-15 and P-32 tracers. Soil Biol. Biochem. 82, 135–142 (2015).
Reich, P. B. et al. Do species and functional groups differ in acquisition and use of C, N and water under varying atmospheric CO2 and N availability regimes? A field test with 16 grassland species. N. Phytol. 150, 435–448 (2001).
Denef, K. et al. Community shifts and carbon translocation within metabolically-active rhizosphere microorganisms in grasslands under elevated CO2. Biogeosciences 4, 769–779 (2007).
Jin, J., Tang, C. X. & Sale, P. The impact of elevated carbon dioxide on the phosphorus nutrition of plants: a review. Ann. Bot. 116, 987–999 (2015).
Lidbury, I. et al. The ‘known’ genetic potential for microbial communities to degrade organic phosphorus is reduced in low-pH soils. Microbiologyopen 6, 1–5 (2017).
Lidbury, I. et al. Niche-adaptation in plant-associated Bacteroidetes favours specialisation in organic phosphorus mineralisation. ISME J. 15, 1040–1055 (2021).
Quirk, J., Andrews, M. Y., Leake, J. R., Banwart, S. A. & Beerling, D. J. Ectomycorrhizal fungi and past high CO2 atmospheres enhance mineral weathering through increased belowground carbon-energy fluxes. Biol. Lett. 10, 20140375 (2014).
Peltzer, D. A. et al. Understanding ecosystem retrogression. Ecol. Monogr. 80, 509529 (2010).
Chapin, F. S., Matson, P. A. & Vitousek, P. M. (eds) in Principles of Terrestrial Ecosystem Ecology 259–296 (Springer, 2011).
Zhu, Q., Riley, W. J. & Tang, J. Y. A new theory of plant-microbe nutrient competition resolves inconsistencies between observations and model predictions. Ecol. Appl. 27, 875–886 (2017).
Finzi, A. C. et al. Increases in nitrogen uptake rather than nitrogen-use efficiency support higher rates of temperate forest productivity under elevated CO2. Proc. Natl Acad. Sci. USA 104, 14014–14019 (2007).
Sulman, B. N., Phillips, R. P., Oishi, A. C., Shevliakova, E. & Pacala, S. W. Microbe-driven turnover offsets mineral-mediated storage of soil carbon under elevated CO2. Nat. Clim. Change 4, 1099–1102 (2014).
Miglietta, F. et al. Spatial and temporal performance of the MiniFACE (free air CO2 enrichment) system on bog ecosystems in northern and central Europe. Environ. Monit. Assess. 66, 107–127 (2001).
Grimshaw, H. M. in Chemical Analysis in Environmental Research (ed. Rowland, A. P.) 92–95 (Abbots Ripton, 1987).
Leake, J. R. The Causes and Effects of Soil Acidification by Calluna vulgaris L (Hull) with Special Reference to the Role of Mycorrhizas. Doctoral thesis, Univ. of Sheffield (1988).
Murphy, J. & Riley, J. P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 27, 31–36 (1962).
Brookes, P. C., Powlson, D. S. & Jenkinson, D. S. Measurement of microbial biomass phosphorus in soil. Soil Biol. Biochem. 14, 319–329 (1982).
Acknowledgements
This work was funded by the Natural Environment Research Council (grant number NE/N0100086/1 to I.P.H. and NE/N010132/1 to G.K.P.) as part of the Phosphorus Limitation And ecosystem responses to Carbon dioxide Enrichment (PLACE) project. We thank Natural England for access to their Wardlow SSSI, S. Taylor (Natural England) for help with monolith extraction and transport and the Peak Park authority for permission to establish the miniFACE experiment within the Peak District National Park. We are grateful to G. McClean for work in establishing the experiment and C. Hook, I. Johnson and E. Paton for P analyses. For the purpose of open access, the author(s) has applied a Creative Commons Attribution (CC BY) licence (where permitted by UKRI, ‘Open Government Licence’ or ‘Creative Commons Attribution No-derivatives (CC BY-ND) licence’ may be stated instead) to any Author Accepted Manuscript version arising.
Author information
Authors and Affiliations
Contributions
I.P.H., G.K.P., J.B.K., J.R.L. and M.R.H. designed the eCO2 experiment. G.K.P., I.P.H., F.M., J.B.K. and C.R.T. installed the FACE system and the mesocosms. J.B.K. and C.R.T. oversaw the operation and maintenance of the experiment. Lab analyses were undertaken by J.B.K. and C.R.T., and J.B.K. performed the data analyses. J.B.K., G.K.P. and I.P.H. wrote the original draft of the manuscript, and all authors contributed to subsequent revisions.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Geoscience thanks Benjamin Turner and Carlo Calfapietra for their contribution to the peer review of this work. Primary Handling Editor: Xujia Jiang, in collaboration with the Nature Geoscience team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
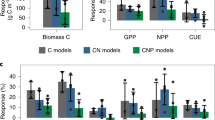
Extended Data Fig. 1 Contrasting aboveground productivity responses to CO2.
Grasslands were exposed to ambient (a- dark green bars and filled circles) or elevated CO2 at 600 ppm (e-light green bars and filled circles), in acidic, (left hand column) and limestone grasslands, (right hand column). Data show means (± SE) in time series (n = 5) and cumulative productivity (n = 5) over the study period. Vertical arrows denote the start of CO2 fumigation.
Extended Data Fig. 2 Soil microbial biomass P (MBP) responses to CO2.
Grasslands were exposed to ambient (a- dark green bars and filled circles) or elevated CO2 at 600 ppm (e-light green bars and filled circles), in acidic, (left hand column) and limestone grasslands, (right hand column). Data show means (± SE) in time series (n = 5) and overall mean MBP (n = 5) over the study period. Vertical arrows denote start of CO2 fumigation.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ben Keane, J., Hartley, I.P., Taylor, C.R. et al. Grassland responses to elevated CO2 determined by plant–microbe competition for phosphorus. Nat. Geosci. 16, 704–709 (2023). https://doi.org/10.1038/s41561-023-01225-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41561-023-01225-z
This article is cited by
-
Elevated CO2 levels promote both carbon and nitrogen cycling in global forests
Nature Climate Change (2024)
-
Belowground Biomass Changed the Regulatory Factors of Soil N2O Funder N and Water Additions in a Temperate Steppe of Inner Mongolia
Journal of Soil Science and Plant Nutrition (2024)
-
Different Response of Arbuscular Mycorrhizal Fungal Communities in Roots and Rhizosphere Soil of Elymus nutans to Long-term Warming in an Alpine Meadow
Journal of Soil Science and Plant Nutrition (2024)
-
Competing for phosphorus
Nature Geoscience (2023)