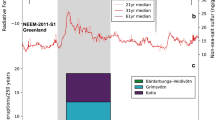
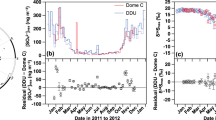
Abstract
Sulfate aerosols affect climate by scattering radiation and by changing the microphysical properties of water clouds. In much of the continental interiors that are overwhelmed by anthropogenic sulfate today, the nature of pre-industrial atmospheric sulfate remains pure speculation, which hampers our ability to quantify anthropogenic perturbation on climate and uncertainties in global climate models. Here we show that sequential leaching and multiple-isotope measurement enabled us to effectively distinguish sulfate of different origins, including pre-industrial atmospheric sulfate, retained in certain outcropping carbonates. Data from two interior sites in northern China show that one of the sulfate endmembers consistently has an unusually positive 17O anomaly (Δʹ17O at ~+1.8‰) and characteristic δ18O (~1–5‰) and δ34S (~5–10‰) values. We interpret this sulfate endmember to be integrated pre-industrial atmospheric sulfate from at least the last a few thousands of years. A triple oxygen isotope enabled GEOS-Chem chemical transport model revealed a higher Δʹ17O value in northern China and the south-western United States in the past, consistent with our data. Overall, pre-industrial atmospheric sulfate aerosol chemistry in the interior of northern China and south-western United States had a higher pH in cloud water, which may have led to a less cloud cover due to cleaner air and coarser aerosol sizes than today.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Code availability
The GEOS-Chem model used in this study is available at https://doi.org/10.5281/zenodo.3403111. The code used to run the model and to analyse the results is not publicly available, but can be provided upon request by contacting S.H. (hattori@nju.edu.cn).
References
Seinfeld, J. H. et al. Improving our fundamental understanding of the role of aerosol−cloud interactions in the climate system. Proc. Natl Acad. Sci. USA 113, 5781–5790 (2016).
Menon, S. Current uncertainties in assessing aerosol effects on climate. Annu. Rev. Environ. Resour. 29, 1–30 (2004).
Matsui, H. Black carbon absorption efficiency under preindustrial and present-day conditions simulated by a size- and mixing-state-resolved global aerosol model. J. Geophys. Res. Atmos. 125, e2019JD032316 (2020).
Hamilton, D. S. et al. Reassessment of pre-industrial fire emissions strongly affects anthropogenic aerosol forcing. Nat. Commun. 9, 3182 (2018).
Carslaw, K. S. et al. Large contribution of natural aerosols to uncertainty in indirect forcing. Nature 503, 67–71 (2013).
Carslaw, K. S. et al. Aerosols in the pre-industrial atmosphere. Curr. Clim. Change Rep. 3, 1–15 (2017).
Rodhe, H. Human impact on the atmospheric sulfur balance. Tellus B. 51, 110–122 (1999).
Alexander, B., Savarino, J., Kreutz, K. J. & Thiemens, M. H. Impact of preindustrial biomass-burning emissions on the oxidation pathways of tropospheric sulfur and nitrogen. J. Geophys. Res. Atmos. 109, D08303/08301–D08303/08308 (2004).
Lelieveld, J., Peters, W., Dentener, F. J. & Krol, M. C. Stability of tropospheric hydroxyl chemistry. J. Geophys. Res. Atmos. https://doi.org/10.1029/2002JD002272 (2002).
Andreae, M. O. Aerosols before pollution. Science 315, 50–51 (2007).
Korhonen, H., Carslaw, K. S., Spracklen, D. V., Mann, G. W. & Woodhouse, M. T. Influence of oceanic dimethyl sulfide emissions on cloud condensation nuclei concentrations and seasonality over the remote Southern Hemisphere oceans: a global model study. J. Geophys. Res. Atmos. https://doi.org/10.1029/2007JD009718 (2008).
Hamilton, D. S. et al. Occurrence of pristine aerosol environments on a polluted planet. Proc. Natl Acad. Sci. USA 111, 18466–18471 (2014).
Kunasek, S. A. et al. Sulfate sources and oxidation chemistry over the past 230 years from sulfur and oxygen isotopes of sulfate in a West Antarctic ice core. J. Geophys. Res. Atmos. https://doi.org/10.1029/2010jd013846 (2010).
Walters, W. W. et al. Assessing the seasonal dynamics of nitrate and sulfate aerosols at the south pole utilizing stable isotopes. J. Geophys. Res. Atmos. 124, 8161–8177 (2019).
Iizuka, Y. et al. A 60 year record of atmospheric aerosol depositions preserved in a high-accumulation dome ice core, Southeast Greenland. J. Geophys. Res. Atmos. 123, 574–589 (2018).
Hill-Falkenthal, J., Priyadarshi, A., Savarino, J. & Thiemens, M. Seasonal variations in 35S and D17O of sulfate aerosols on the Antarctic Plateau. J. Geophys. Res. Atmos. 118, 9444–9455 (2013).
Wynn, P. M., Loader, N. J. & Fairchild, I. J. Interrogating trees for isotopic archives of atmospheric sulphur deposition and comparison to speleothem records. Environ. Pollut. 187, 98–105 (2014).
Peng, Y. et al. Widespread contamination of carbonate-associated sulfate by present-day secondary atmospheric sulfate: evidence from triple oxygen isotopes. Geology 42, 815–818 (2014).
Bao, H. Sulfate: a time capsule for Earth’s O2, O3, and H2O. Chem. Geol. 395, 108–118 (2015).
Crockford, P. W. et al. Claypool continued: extending the isotopic record of sedimentary sulfate. Chem. Geol. 513, 200–225 (2019).
Pingitore, N. E., Meitzner, G. & Love, K. M. Identification of sulfate in natural carbonates by x-ray absorption spectroscopy. Geochim. Cosmochim. Acta 59, 2477–2483 (1995).
Zuo, P. et al. Reviews of the Mesoproterozoic to Neoproterozoic sedimentary sequences and new constraints on the tectono-sedimentary evolution of the southern margin of the North China Craton. J. Asian Earth Sci. 179, 416–429 (2019).
Ma, H. et al. Sulfur and oxygen isotopic compositions of carbonate associated sulfate (CAS) of Cambrian ribbon rocks: implications for the constraints on using CAS to reconstruct seawater sulfate sulfur isotopic compositions. Chem. Geol. 580, 120369 (2021).
Wang, K. et al. Isotopic constraints on atmospheric sulfate formation pathways in the Mt. Everest region, southern Tibetan Plateau. Atmos. Chem. Phys. 21, 8357–8376 (2021).
Smith, S. J. et al. Anthropogenic sulfur dioxide emissions: 1850–2005. Atmos. Chem. Phys. 11, 1101–1116 (2011).
Mayewski, P. A. et al. Sulfate and nitrate concentrations from a South Greenland ice core. Science 232, 975–977 (1986).
Kreidenweis, S. M. et al. Modification of aerosol mass and size distribution due to aqueous-phase SO2 oxidation in clouds: comparisons of several models. J. Geophys. Res. Atmos. https://doi.org/10.1029/2002JD002697 (2003)
Krankowsky, D., Bartecki, F., Klees, G. G., Mauersberger, K. & Schellenbach, K. Measurement of heavy isotope enrichment in tropospheric ozone. Geophys. Res. Lett. 22, 1713–1716 (1995).
Sofen, E. D., Alexander, B. & Kunasek, S. A. The impact of anthropogenic emissions on atmospheric sulfate production pathways, oxidants, and ice core D17O(SO42−). Atmos. Chem. Phys. 11, 3565–3578 (2011).
Hattori, S. et al. Isotopic evidence for acidity-driven enhancement of sulfate formation after SO2 emission control. Sci. Adv. 7, eabd4610 (2021).
Calhoun, J. A., Bates, T. S. & Charlson, R. J. Sulfur isotope measurements of submicrometer sulfate aerosol particles over the Pacific Ocean. Geophys. Res. Lett. 18, 1877–1880 (1991).
Amrani, A., Said-Ahmad, W., Shaked, Y. & Kiene, R. P. Sulfur isotope homogeneity of oceanic DMSP and DMS. Proc. Natl Acad. Sci. USA 110, 18413–18418 (2013).
Patris, N. et al. First sulfur isotope measurements in central Greenland ice cores along the preindustrial and industrial periods. J. Geophys. Res. Atmos. 107, 4115 (2002).
Rozanski, K., Araguas-Araguas, L. & Gonfiantini, R. in Climate Change in Continental Isotopic Records Vol. 78 (eds Swart, P. K. et al.) 1–36 (American Geophysical Union Geophysical Monograph, 1993).
Herman, R. L. et al. Aircraft validation of Aura Tropospheric Emission Spectrometer retrievals of HDO/H2O. Atmos. Meas. Tech. 7, 3127–3138 (2014).
Dyroff, C. et al. Airborne in situ vertical profiling of HDO/H216O in the subtropical troposphere during the MUSICA remote sensing validation campaign. Atmos. Meas. Tech. 8, 2037–2049 (2015).
Spracklen, D. V. & Rap, A. Natural aerosol–climate feedbacks suppressed by anthropogenic aerosol. Geophys. Res. Lett. 40, 5316–5319 (2013).
Rap, A. et al. Natural aerosol direct and indirect radiative effects. Geophys. Res. Lett. 40, 3297–3301 (2013).
Turnock, S. T. et al. The impact of changes in cloud water pH on aerosol radiative forcing. Geophys. Res. Lett. 46, 4039–4048 (2019).
Li, X. Q., Bao, H., Gan, Y. Q., Zhou, A. G. & Liu, Y. D. Multiple oxygen and sulfur isotope compositions of secondary atmospheric sulfate in a mega-city in central China. Atmos. Environ. 81, 591–599 (2013).
Jenkins, K. A. & Bao, H. Multiple oxygen and sulfur isotope compositions of atmospheric sulfate in Baton Rouge, LA, USA. Atmos. Environ. 40, 4528–4537 (2006).
Jedrysek, M. O. Oxygen and sulphur isotope dynamics in the SO42− of an urban precipitation. Water Air Soil Pollut. 117, 15–25 (2000).
Jamieson, R. E. & Wadleigh, M. A. Tracing sources of precipitation sulfate in eastern Canada using stable isotopes and trace metals. J. Geophys. Res. Atmos. 105, 20549–20556 (2000).
Wadleigh, M. A., Schwarcz, H. P. & Kramer, J. R. Isotopic evidence for the origin of sulfate in coastal rain. Tellus B 48B, 44–59 (1996).
McCabe, J. R., Savarino, J., Alexander, B., Gong, S. L. & Thiemens, M. H. Isotopic constraints on non-photochemical sulfate production in the Arctic winter. Geophys. Res. Lett. https://doi.org/10.1029/2005gl025164 (2006).
Bao, H. & Marchant, D. R. Quantifying sulfate components and their variations in soils of the McMurdo Dry Valleys, Antarctica. J. Geophys. Res. Atmos. https://doi.org/10.1029/2005JD006669 (2006).
Genot, I. et al. Oxygen and sulfur mass-independent isotopic signatures in black crusts: the complementary negative Δ33S reservoir of sulfate aerosols? Atmos. Chem. Phys. 20, 4255–4273 (2020).
Bao, H. M. Purifying barite for oxygen isotope measurement by dissolution and reprecipitation in a chelating solution. Anal. Chem. 78, 304–309 (2006).
Bey, I. et al. Global modeling of tropospheric chemistry with assimilated meteorology: model description and evaluation. J. Geophys. Res. Atmos. 106, 23073–23095 (2001).
Alexander, B. et al. Isotopic constraints on the formation pathways of sulfate aerosol in the marine boundary layer of the subtropical northeast Atlantic Ocean. J. Geophys. Res. Atmos. https://doi.org/10.1029/2011JD016773 (2012).
Chen, Q. et al. Sulfate production by reactive bromine: implications for the global sulfur and reactive bromine budgets. Geophys. Res. Lett. 44, 7069–7078 (2017).
Hoesly, R. M. et al. Historical (1750–2014) anthropogenic emissions of reactive gases and aerosols from the Community Emissions Data System (CEDS). Geosci. Model Dev. 11, 369–408 (2018).
van Marle, M. J. E. et al. Historic global biomass burning emissions for CMIP6 (BB4CMIP) based on merging satellite observations with proxies and fire models (1750–2015). Geosci. Model Dev. 10, 3329–3357 (2017).
Meinshausen, M. et al. Historical greenhouse gas concentrations for climate modelling (CMIP6). Geosci. Model Dev. 10, 2057–2116 (2017).
Ishino, S. et al. Regional characteristics of atmospheric sulfate formation in east antarctica imprinted on 17O-excess signature. J. Geophys. Res. Atmos. 126, e2020JD033583 (2021).
Acknowledgements
Financial support is provided by the National Key Research and Development Program of China (2022YFF0800303), the National Natural Science Foundation of China (42173001, 41490635 and 41672334), Nanjing University Start-up fund (to H.B.), MEXT/JSPS KAKENHI (JP16H0584 and JP20H04305), Fundamental Research Funds for the Central Universities (0206/14380150, 0206/14380185 and 0206/14380174), Natural Science Foundation of Henan (202300410178) and Key Scientific Research Project in Colleges and Universities of Henan (18A170001). We thank S. Zhai and S. Ishino for providing support for handling the GEOS-Chem model.
Author information
Authors and Affiliations
Contributions
H.B., Y.P. and S.H. conceived the research idea; Y.P., P.Z. and H.M. did the analytical treatments; S.H. contributed to the model simulations; and H.B. wrote the paper with writing contributions from all co-authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Geoscience thanks Theodore Present and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor(s): James Super, in collaboration with the Nature Geoscience team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Peng, Y., Hattori, S., Zuo, P. et al. Record of pre-industrial atmospheric sulfate in continental interiors. Nat. Geosci. 16, 619–624 (2023). https://doi.org/10.1038/s41561-023-01211-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41561-023-01211-5