Abstract
Soil represents the largest terrestrial carbon pool, and it liberates massive amounts of carbon dioxide (CO2) to the atmosphere via respiration, which can influence global carbon cycle. In recent decades, anthropogenic activities have dramatically increased the rates of atmospheric nitrogen (N) deposition worldwide, but our current understanding of soil respiration dynamics in anthropogenic N-deposition environments remains poor. Here we monitored soil CO2 emission rates monthly following 9–13 years of N-addition treatments in three tropical forests in southern China. We found a three-phase pattern of soil CO2 emission (insignificant changes–dramatic decline–insignificant changes) in three tropical forests and across three N-addition gradients. During the course of the experiments, N addition reduced a total cumulative amount of 6.53–9.06 MgCO2 ha–1 with the efficiency of 5.80–13.13 MgCO2 Mg N–1. The mechanisms underlying the temporal patterns of soil respiration were related to the lack of plant and microbial responses (phase 1), the reduction in fine root and microbial biomass due to soil acidification (phase 2) and the reorganization of plant and microbial community (phase 3). These findings advance our understanding of soil respiration dynamics and support prediction of long-term soil C fluxes in tropical forests in the context of N deposition.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data supporting the findings of this study are available from Figshare Digital Repository: https://figshare.com/articles/dataset/Dataset_Mianhai_Zheng_et_al_NG_2022/21291888.
Code availability
No custom code was used in this study.
References
Arneth, A. et al. Terrestrial biogeochemical feedbacks in the climate system. Nat. Geosci. 3, 525–532 (2010).
Adoption of the Paris Agreement FCCC/CP/2015/L.9/Rev.1 (UNFCC, 2015).
IPCC Special Report on Climate Change and Land (eds Shukla, P. R. et al.) (IPCC, 2019).
Oertel, C., Matschullat, J., Zurba, K., Zimmermann, F. & Erasmi, S. Greenhouse gas emissions from soils—a review. Geochemistry 76, 327–352 (2016).
Schlesinger, W. H. & Bernhardt, E. S. Biogeochemistry: An Analysis of Global Change 3rd edn (Elsevier, 2013).
Harris, N. L. et al. Global maps of twenty-first century forest carbon fluxes. Nat. Clim. Change 11, 234–240 (2021).
Ackerman, D., Millet, D. B. & Chen, X. Global estimates of inorganic nitrogen deposition across four decades. Glob. Biogeochem. Cycles 33, 100–107 (2019).
Du, E. Rise and fall of nitrogen deposition in the United States. Proc. Natl Acad. Sci. USA 113, E3594–E3595 (2016).
Schmitz, A. et al. Responses of forest ecosystems in Europe to decreasing nitrogen deposition. Environ. Pollut. 244, 980–994 (2019).
Hietz, P. et al. Long-term change in the nitrogen cycle of tropical forests. Science 334, 664–666 (2011).
Fang, Y. T., Gundersen, P., Mo, J. M. & Zhu, W. X. Input and output of dissolved organic and inorganic nitrogen in subtropical forests of South China under high air pollution. Biogeosciences 5, 339–352 (2008).
Yu, G. et al. Stabilization of atmospheric nitrogen deposition in China over the past decade. Nat. Geosci. 12, 424–429 (2019).
Liu, L. L. & Greaver, T. L. A global perspective on belowground carbon dynamics under nitrogen enrichment. Ecol. Lett. 13, 819–828 (2010).
LeBauer, D. S. & Treseder, K. K. Nitrogen limitation of net primary productivity in terrestrial ecosystems is globally distributed. Ecology 89, 371–379 (2008).
Reich, P. B. et al. Scaling of respiration to nitrogen in leaves, stems and roots of higher land plants. Ecol. Lett. 11, 793–801 (2008).
Cornwell, W. K. et al. Plant species traits are the predominant control on litter decomposition rates within biomes worldwide. Ecol. Lett. 11, 1065–1071 (2008).
Mo, J. et al. Nitrogen addition reduces soil respiration in a mature tropical forest in southern China. Glob. Change Biol. 14, 403–412 (2008).
Janssens, I. A. et al. Reduction of forest soil respiration in response to nitrogen deposition. Nat. Geosci. 3, 315–322 (2010).
Zhong, Y., Yan, W. & Shangguan, Z. The effects of nitrogen enrichment on soil CO2 fluxes depending on temperature and soil properties. Glob. Ecol. Biogeogr. 25, 475–488 (2016).
Deng, L. et al. Soil GHG fluxes are altered by N deposition: new data indicate lower N stimulation of the N2O flux and greater stimulation of the calculated C pools. Glob. Change Biol. 26, 2613–2629 (2020).
Hagedorn, F., Kammer, A., Schmidt, M. W. I. & Goodale, C. L. Nitrogen addition alters mineralization dynamics of 13C-depleted leaf and twig litter and reduces leaching of older DOC from mineral soil. Glob. Change Biol. 18, 1412–1427 (2012).
Du, Y. et al. Different types of nitrogen deposition show variable effects on the soil carbon cycle process of temperate forests. Glob. Change Biol. 20, 3222–3228 (2014).
Yan, T. et al. Negative effect of nitrogen addition on soil respiration dependent on stand age: evidence from a 7-year field study of larch plantations in northern China. Agr. For. Meteorol. 262, 24–33 (2018).
Xing, A. et al. Nonlinear responses of ecosystem carbon fluxes to nitrogen deposition in an old-growth boreal forest. Ecol. Lett. 25, 77–78 (2021).
Melillo, J. et al. Long-term pattern and magnitude of soil carbon feedback to the climate system in a warming world. Science 358, 101–105 (2017).
Gao, Q. et al. Stimulation of soil respiration by elevated CO2 is enhanced under nitrogen limitation in a decade-long grassland study. Proc. Natl Acad. Sci. USA 117, 33317–33324 (2020).
Liu, X. J. et al. Nitrogen deposition and its ecological impact in China: an overview. Environ. Pollut. 159, 2251–2264 (2011).
Zhu, F. F., Yoh, M., Gilliam, F. S., Lu, X. K. & Mo, J. M. Nutrient limitation in three lowland tropical forests in southern China receiving high nitrogen deposition: insights from fine root responses to nutrient additions. PLoS ONE 8, e82661 (2013).
Wang, C. et al. Responses of soil microbial community to continuous experimental nitrogen additions for 13 years in a nitrogen-rich tropical forest. Soil Biol. Biochem. 121, 103–112 (2018).
Priess, J. & Fölster, H. Microbial properties and soil respiration in submontane forests of Venezuelian Guyana: characteristics and response to fertilizer treatments. Soil Biol. Biochem. 33, 503–509 (2001).
He, T., Wang, Q., Wang, S. & Zhang, F. Nitrogen addition altered the effect of belowground C allocation on soil respiration in a subtropical forest. PLoS ONE 11, e0155881 (2016).
Fan, H. et al. Nitrogen deposition promotes ecosystem carbon accumulation by reducing soil carbon emission in a subtropical forest. Plant Soil 379, 361–371 (2014).
Zheng, M. et al. Effects of nitrogen and phosphorus additions on nitrous oxide emission in a nitrogen-rich and two nitrogen-limited tropical forests. Biogeosciences 13, 3503–3517 (2016).
Lu, X. et al. Nitrogen deposition accelerates soil carbon sequestration in tropical forests. Proc. Natl Acad. Sci. USA 118, e2020790118 (2021).
Zhou, G. Y. et al. Old-growth forests can accumulate carbon in soils. Science 314, 1417–1417 (2006).
Tian, J. et al. Long-term nitrogen addition modifies microbial composition and functions for slow carbon cycling and increased sequestration in tropical forest soil. Glob. Change Biol. 25, 3267–3281 (2019).
Huang, N. et al. Spatial and temporal variations in global soil respiration and their relationships with climate and land cover. Sci. Adv. 6, eabb8508 (2020).
Lu, X. K. et al. Effect of simulated N deposition on soil exchangeable cations in three forest types of subtropical China. Pedosphere 19, 189–198 (2009).
Fang, Y., Gundersen, P., Mo, J. & Zhu, W. Nitrogen leaching in response to increased nitrogen inputs in subtropical monsoon forests in southern China. For. Ecol. Manage. 257, 332–342 (2009).
Chen, X. M. et al. Effects of nitrogen deposition on soil organic carbon fractions in the subtropical forest ecosystems of S. China. J. Plant Nutr. Soil Sci. 175, 947–953 (2012).
Fang, H. J. et al. 13C abundance, water-soluble and microbial biomass carbon as potential indicators of soil organic carbon dynamics in subtropical forests at different successional stages and subject to different nitrogen loads. Plant Soil 320, 243–254 (2009).
Liu, L. et al. Effects of nitrogen and phosphorus additions on soil microbial biomass and community structure in two reforested tropical forests. Sci. Rep. 5, 14378–14378 (2014).
Chen, H. et al. Nitrogen saturation in humid tropical forests after 6 years of nitrogen and phosphorus addition: hypothesis testing. Funct. Ecol. 30, 305–313 (2015).
Lu, X., Mao, Q., Gilliam, F. S., Luo, Y. & Mo, J. Nitrogen deposition contributes to soil acidification in tropical ecosystems. Glob. Change Biol. 20, 3790–3801 (2014).
Mao, Q. G. Impacts of Long-Term Nitrogen and Phosphorus Addition on Understory Plant Diversity in Subtropical Forests in Southern China. Doctoral Thesis, Univ. Chinese Academy of Sciences (2017).
Xing, A. J. et al. High-level nitrogen additions accelerate soil respiration reduction over time in a boreal forest. Ecol. Lett. https://doi.org/10.1111/ele.14065 (2022).
Cao, J. et al. Plant–bacteria–soil response to frequency of simulated nitrogen deposition has implications for global ecosystem change. Funct. Ecol. 34, 723–734 (2020).
Mo, J. M., Brown, S., Peng, S. L. & Kong, G. H. Nitrogen availability in disturbed, rehabilitated and mature forests of tropical China. For. Ecol. Manage. 175, 573–583 (2003).
Huang, Z. L., Ding, M. M., Zhang, Z. P. & Yi, W. M. The hydrological processes and nitrogen dynamics in a monsoon evergreen broad-leafed forest of Dinghushan. Acta Phytoecol. Sin. 18, 194–199 (1994).
Wright, R. F. & Rasmussen, L. Introduction to the NITREX and EXMAN projects. For. Ecol. Manage. 101, 1–7 (1998).
Gundersen, P. et al. Impact of nitrogen deposition on nitrogen cycling in forests: a synthesis of NITREX data. For. Ecol. Manage. 101, 37–55 (1998).
Aber, J. D. et al. Plant and soil responses to chronic nitrogen additions at the Harvard Forest, Massachusetts. Ecol. Appl. 3, 156–166 (1993).
Cleveland, C. C. & Townsend, A. R. Nutrient additions to a tropical rain forest drive substantial soil carbon dioxide losses to the atmosphere. Proc. Natl Acad. Sci. USA 103, 10316–10321 (2006).
Song, X. et al. Nitrogen addition increased CO2 uptake more than non-CO2 greenhouse gases emissions in a Moso bamboo forest. Sci. Adv. 6, eaaw5790 (2020).
Lu, X. et al. Long-term nitrogen addition decreases carbon leaching in nitrogen-rich forest ecosystems. Biogeosciences 10, 3931–3941 (2013).
Ackerman, D., Millet, D. B. & Chen, X. Global estimates of inorganic nitrogen deposition across four decades. Glob. Biogeochem. Cycles 33, 100–107 (2019).
Tang, X., Liu, S., Zhou, G., Zhang, D. & Zhou, C. Soil–atmospheric exchange of CO2, CH4, and N2O in three subtropical forest ecosystems in southern China. Glob. Change Biol. 12, 546–560 (2006).
Lei, J. et al. Temporal changes in global soil respiration since 1987. Nat. Commun. 12, 403 (2021).
Acknowledgements
This study was financially supported by the National Natural Science Foundation of China (41731176, 42077311, 31901164, 42173077), Youth Innovation Promotion Association CAS (2021346), Young Elite Scientists Sponsorship Program by ESC (STQT2020A02) and Guangdong Basic and Applied Basic Research Foundation (2019A1515011821).
Author information
Authors and Affiliations
Contributions
M.Z., J.M. and W.Z. conceived the original idea. M.Z., T.Z., X.L. S.W. and W.Z. performed the experiment. M.Z. collected soil, plant and microbial data and ran the statistical analyses. M.Z. wrote the first draft. M.Z., Y.L., J.L., Q.Y., J.H., Q.M., J.M. and W.Z. discussed and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Geoscience thanks Jens-Arne Subke and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Xujia Jiang, in collaboration with the Nature Geoscience team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
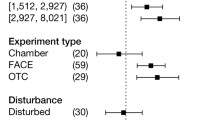
Extended Data Fig. 1 Global meta-analysis of the N-addition effects on soil CO2 flux in 62 forest sites.
Distribution of the number of observation in different experimental periods (a). Distribution of the number of observation in different measurement time point after N treatment (b). Response ratios of soil CO2 flux to N addition in different years after N-addition treatment (c). Response ratios of soil CO2 flux to N addition in different types of forests and the total response ratios (d). Solid circles and error bars represent weighted mean response ratios and 95% confidence intervals, respectively. The numbers under the solid circles in (c, d) represent sample sizes (n values). Data were collected from the literatures published from 1995 to 2021.
Extended Data Fig. 2
Statistical significance of repeated-measures analysis of variance for soil respiration measured in different years with the factors of forest type and N-addition treatment.
Extended Data Fig. 3 Mean annual soil temperature in the control and N-addition plots of the primary forest (a-b), secondary forest (c-d), and planted forest (e-f).
Columns and error bars represent means and standard errors (n = 3), respectively. The asterisks indicate the years in which statistical significance (P < 0.05; one-way analysis of variance followed by Tukey’s HSD test for Exp1 and independent-sample t-test for Exp2) is detected between the control and N-addition plots. C: control; L: low N addition; M: medium N addition; H: high N addition.
Extended Data Fig. 4 Mean annual soil moisture in the control and N-addition plots of the primary forest (a-b), secondary forest (c-d), and planted forest (e-f).
Columns and error bars represent means and standard errors (n = 3), respectively. The asterisks indicate the years in which statistical significance (P < 0.05; one-way analysis of variance followed by Tukey’s HSD test for Exp1 and independent-sample t-test for Exp2) is detected between the control and N-addition plots. C: control; L: low N addition; M: medium N addition; H: high N addition.
Extended Data Fig. 5 The relationships between soil temperature and time in the primary, secondary, and planted forests.
Linear regression models of soil temperature (in the control, low N-addition, and medium N-addition plots) against time from January 2011 to December 2019 (a, c, e). Linear regression models of soil temperature (in the control and high N-addition plots) against time from February 2007 to December 2019 (b, d, f). C: control; L: low N addition; M: medium N addition; H: high N addition.
Extended Data Fig. 6 The relationships between soil moisture and time in the primary, secondary, and planted forests.
Linear regression models of soil moisture (in the control, low N-addition, and medium N-addition plots) against time from January 2011 to December 2019 (a, c, e). Linear regression models of soil moisture (in the control and high N-addition plots) against time from February 2007 to December 2019 (b, d, f). C: control; L: low N addition; M: medium N addition; H: high N addition.
Extended Data Fig. 7 Three-dimensional surface models of the relationships among soil respiration, soil temperature, and soil moisture in the three forests.
Polynomial regression models of soil respiration against soil temperature and moisture (in combination of the control, low N-addition, and medium N-addition plots; Exp 1) from 2011 to 2019 (a, c, e). Polynomial regression models of soil respiration against soil temperature and moisture (in combination of the control and high N-addition plots: Exp 2) from 2007 to 2019 (b, d, f). C: control; L: low N addition; M: medium N addition; H: high N addition.
Extended Data Fig. 8 The parameters and statistics of soil respiration to soil temperature (exponential regression models) and moisture (logarithmic regression models) in the control and N-addition plots in the studied forests.
Experiment 1 (Exp 1): N treatments started from 2003, and soil respiration, temperature, and moisture were monitored from 2011 to 2019. Experiment 2 (Exp 2): N treatments started from 2007, and soil respiration, temperature, and moisture were monitored from 2007 to 2019. Rs: soil respiration; T: soil temperature; M: soil moisture.
Extended Data Fig. 9 Exponential regression models of soil respiration against soil temperature in the primary, secondary, and planted forests.
Exponential regression models of soil CO2 flux against soil temperature in the control, low N-addition, and medium N-addition plots in 2011 − 2019 (a, c, e). Exponential regression models of soil CO2 flux against soil temperature in the control and high N-addition plots in 2007 − 2019 (b, d, f). C: control; L: low N addition; M: medium N addition; H: high N addition.
Extended Data Fig. 10 Logarithmic regression models of soil respiration against soil moisture in the primary, secondary, and planted forests.
Logarithmic regression models of soil CO2 flux against soil moisture in the control, low N-addition, and medium N-addition plots in 2011 − 2019 (a, c, e). Logarithmic regression models of soil CO2 flux against soil moisture in the control and high N-addition plots in 2007 − 2019 (b, d, f). C: control; L: low N addition; M: medium N addition; H: high N addition.
Supplementary information
Supplementary Information
Supplementary Figs. 1–4, Tables 1 and 2 and references.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zheng, M., Zhang, T., Luo, Y. et al. Temporal patterns of soil carbon emission in tropical forests under long-term nitrogen deposition. Nat. Geosci. 15, 1002–1010 (2022). https://doi.org/10.1038/s41561-022-01080-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41561-022-01080-4