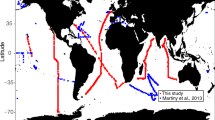
Abstract
Dissolved organic phosphorus (DOP) has a dual role in the surface ocean as both a product of primary production and as an organic nutrient that fuels primary production and nitrogen fixation, especially in oligotrophic gyres. Although poorly constrained, the geographic distribution and environmental controls of surface ocean DOP concentrations influence the distributions and rates of primary production and nitrogen fixation in the global ocean. Here we pair DOP concentration measurements with a metric of phosphate stress, satellite-based chlorophyll a concentrations and a satellite-based iron stress proxy to explore their relationship with upper 50 m DOP stocks. Our results suggest that phosphate and iron stress work together to control surface ocean DOP concentrations at basin scales. Specifically, upper 50 m DOP stocks decrease with increasing phosphate stress, while alleviated iron stress leads to either surface DOP accumulation or loss depending on phosphate availability. Our work extends the relationship between DOP concentrations and phosphate availability to the global ocean, suggests a linkage between marine phosphorus cycling and iron availability and establishes a predictive framework for DOP distributions and their use as an organic nutrient source that supports global ocean fertility.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
Original DOP data used in this study can be found and freely accessed in the DOPv2021 database archived on the BCO DMO website (https://www.bco-dmo.org/dataset/855139) or in the Woods Hole Open Access Server (https://doi.org/10.26008/1912/bco-dmo.855139.2)61. The Level-3 satellite product can be downloaded from the NASA OceanColor website (https://oceancolor.gsfc.nasa.gov/l3/).
Code availability
Code and data used to reproduce Figs. 1 and 2 are archived at https://github.com/zliangocean/DOP.
References
Björkman, K. M. & Karl, D. M. Bioavailability of dissolved organic phosphorus in the euphotic zone at Station ALOHA, North Pacific Subtropical Gyre. Limnol. Oceanogr. 48, 1049–1057 (2003).
Mather, R. L. et al. Phosphorus cycling in the North and South Atlantic Ocean subtropical gyres. Nat. Geosci. 1, 439–443 (2008).
Lomas, M. W. et al. Sargasso Sea phosphorus biogeochemistry: an important role for dissolved organic phosphorus (DOP). Biogeosciences 7, 695–710 (2010).
Dyhrman, S. T. et al. Phosphonate utilization by the globally important marine diazotroph Trichodesmium. Nature 439, 68–71 (2006).
Dyhrman, S. T. & Ruttenberg, K. C. Presence and regulation of alkaline phosphatase activity in eukaryotic phytoplankton from the coastal ocean: implications for dissolved organic phosphorus remineralization. Limnol. Oceanogr. 51, 1381–1390 (2006).
Orchard, E. D., Ammerman, J. W., Lomas, M. W. & Dyhrman, S. T. Dissolved inorganic and organic phosphorus uptake in Trichodesmium and the microbial community: the importance of phosphorus ester in the Sargasso Sea. Limnol. Oceanogr. 55, 1390–1399 (2010).
Sato, M., Sakuraba, R. & Hashihama, F. Phosphate monoesterase and diesterase activities in the North and South Pacific Ocean. Biogeosciences 10, 7677–7688 (2013).
Reynolds, S., Mahaffey, C., Roussenov, V. & Williams, R. G. Evidence for production and lateral transport of dissolved organic phosphorus in the eastern subtropical North Atlantic. Global Biogeochem. Cycles 28, 805–824 (2014).
Diaz, J. M. et al. Dissolved organic phosphorus utilization by phytoplankton reveals preferential degradation of polyphosphates over phosphomonoesters. Front. Mar. Sci. https://doi.org/10.3389/fmars.2018.00380 (2018).
Duhamel, S. et al. Phosphorus as an integral component of global marine biogeochemistry. Nat. Geosci. 14, 359–368 (2021).
Letscher, R. T. & Moore, J. K. Preferential remineralization of dissolved organic phosphorus and non-Redfield DOM dynamics in the global ocean: impacts on marine productivity, nitrogen fixation, and carbon export. Global Biogeochem. Cycles 29, 325–340 (2015).
Carlson, C. A. & Hansell, D. A. in Biogeochemistry of Marine Dissolved Organic Matter 2nd edn (eds. Hansell, D. A. & Carlson, C. A.) 65–126, Ch. 3 DOM Sources, Sinks, Reactivity, and Budgets (Academic Press, 2015); https://doi.org/10.1016/B978-0-12-405940-5.00003-0
Browning, T. J. et al. Iron limitation of microbial phosphorus acquisition in the tropical North Atlantic. Nat. Commun. 8, 15465 (2017).
Mahaffey, C., Reynolds, S., Davis, C. E. & Lohan, M. C. Alkaline phosphatase activity in the subtropical ocean: insights from nutrient, dust and trace metal addition experiments. Front. Mar. Sci. https://doi.org/10.3389/fmars.2014.00073 (2014)
Deutsch, C., Sarmiento, J. L., Sigman, D. M., Gruber, N. & Dunne, J. P. Spatial coupling of nitrogen inputs and losses in the ocean. Nature 445, 163–167 (2007).
Behrenfeld, M. J. et al. Satellite-detected fluorescence reveals global physiology of ocean phytoplankton. Biogeosciences 6, 779–794 (2009).
Church, M. J., Ducklow, H. W. & Karl, D. M. Multiyear increases in dissolved organic matter inventories at Station ALOHA in the North Pacific Subtropical Gyre. Limnol. Oceanogr. 47, 1–10 (2002).
Quigg, A. et al. The evolutionary inheritance of elemental stoichiometry in marine phytoplankton. Nature 425, 291–294 (2003).
Bronk, D. A. & Ward, B. B. Gross and net nitrogen uptake and DON release in the euphotic zone of Monterey Bay, California. Limnol. Oceanogr. 44, 573–585 (1999).
Hansell, D. A. & Carlson, C. A. Biogeochemistry of total organic carbon and nitrogen in the Sargasso Sea: control by convective overturn. Deep Sea Res. 2 Top. Stud. Oceanogr. 48, 1649–1667 (2001).
Letscher, R. T., Hansell, D. A., Carlson, C. A., Lumpkin, R. & Knapp, A. N. Dissolved organic nitrogen in the global surface ocean: distribution and fate. Global Biogeochem. Cycles 27, 141–153 (2013).
Knapp, A. N., Casciotti, K. L. & Prokopenko, M. G. Dissolved organic nitrogen production and consumption in eastern tropical South Pacific surface waters. Global Biogeochem. Cycles 32, 769–783 (2018).
Raimbault, P., Garcia, N. & Cerutti, F. Distribution of inorganic and organic nutrients in the South Pacific Ocean – evidence for long-term accumulation of organic matter in nitrogen-depleted waters. Biogeosciences 5, 281–298 (2008).
Garcia, H. E. et al. World Ocean Atlas 2013. Volume 4: Dissolved Inorganic Nutrients (Phosphate, Nitrate, Silicate) (eds Levitus, S., Mishonov, A.) NOAA atlas NESDIS series No. 76 (NOAA, 2013).
DeVries, T., Deutsch, C., Primeau, F., Chang, B. & Devol, A. Global rates of water-column denitrification derived from nitrogen gas measurements. Nat. Geosci. 5, 547–550 (2012).
Sohm, J. A., Mahaffey, C. & Capone, D. G. Assessment of relative phosphorus limitation of Trichodesmium spp. in the North Pacific, North Atlantic, and the north coast of Australia. Limnol. Oceanogr. 53, 2495–2502 (2008).
Browning, T. J., Bouman, H. A. & Moore, C. M. Satellite-detected fluorescence: decoupling nonphotochemical quenching from iron stress signals in the South Atlantic and Southern Ocean. Global Biogeochem. Cycles 28, 510–524 (2014).
Westberry, T. K. et al. Annual cycles of phytoplankton biomass in the subarctic Atlantic and Pacific Ocean. Global Biogeochem. Cycles 30, 175–190 (2016).
Hopwood, M. J. et al. Non-linear response of summertime marine productivity to increased meltwater discharge around Greenland. Nat. Commun. 9, 3256 (2018).
Larkin, A. A. et al. Subtle biogeochemical regimes in the Indian Ocean revealed by spatial and diel frequency of Prochlorococcus haplotypes. Limnol. Oceanogr. 65, S220–S232 (2020).
Hashihama, F. et al. Biogeochemical controls of particulate phosphorus distribution across the oligotrophic subtropical Pacific Ocean. Global Biogeochem. Cycles 34, e2020GB006669 (2020).
Mahowald, N. M. et al. Atmospheric global dust cycle and iron inputs to the ocean. Global Biogeochem. Cycles https://doi.org/10.1029/2004GB002402 (2005)
Guieu, C. et al. Iron from a submarine source impacts the productive layer of the Western Tropical South Pacific (WTSP). Sci. Rep. 8, 9075 (2018).
Carpenter, E. J., Subramaniam, A. & Capone, D. G. Biomass and primary productivity of the cyanobacterium Trichodesmium spp. in the tropical N Atlantic ocean. Deep Sea Res. 1 Oceanogr. Res. Pap. 51, 173–203 (2004).
Capone, D. G. et al. Nitrogen fixation by Trichodesmium spp.: an important source of new nitrogen to the tropical and subtropical North Atlantic Ocean. Global Biogeochem. Cycles https://doi.org/10.1029/2004GB002331 (2005)
Knapp, A. N. et al. Distribution and rates of nitrogen fixation in the western tropical South Pacific Ocean constrained by nitrogen isotope budgets. Biogeosciences 15, 2619–2628 (2018).
Caffin, M. et al. N2 fixation as a dominant new N source in the western tropical South Pacific Ocean (OUTPACE cruise). Biogeosciences 15, 2565–2585 (2018).
Van Mooy, B. A. S. et al. Quorum sensing control of phosphorus acquisition in Trichodesmium consortia. ISME J. 6, 422–429 (2012).
Behrenfeld, M. J. et al. Controls on tropical Pacific Ocean productivity revealed through nutrient stress diagnostics. Nature 442, 1025–1028 (2006).
Schrader, P. S., Milligan, A. J. & Behrenfeld, M. J. Surplus photosynthetic antennae complexes underlie diagnostics of iron limitation in a cyanobacterium. PLoS ONE 6, e18753 (2011).
Chappell, P. D., Moffett, J. W., Hynes, A. M. & Webb, E. A. Molecular evidence of iron limitation and availability in the global diazotroph Trichodesmium. ISME J. 6, 1728–1739 (2012).
Moreno, C. M., Gong, W., Cohen, N. R., DeLong, K. & Marchetti, A. Interactive effects of iron and light limitation on the molecular physiology of the Southern Ocean diatom Fragilariopsis kerguelensis. Limnol. Oceanogr. 65, 1511–1531 (2020).
Hu, C. et al. Dynamic range and sensitivity requirements of satellite ocean color sensors: learning from the past. Appl. Opt. 51, 6045–6062 (2012).
Huot, Y., Franz, B. A. & Fradette, M. Estimating variability in the quantum yield of Sun-induced chlorophyll fluorescence: a global analysis of oceanic waters. Remote Sens. Environ. 132, 238–253 (2013).
Lin, H. et al. The fate of photons absorbed by phytoplankton in the global ocean. Science 351, 264–267 (2016).
Foreman, R. K., Björkman, K. M., Carlson, C. A., Opalk, K. & Karl, D. M. Improved ultraviolet photo-oxidation system yields estimates for deep-sea dissolved organic nitrogen and phosphorus. Limnol. Oceanogr. Methods 17, 277–291 (2019).
Ustick, L. J. et al. Metagenomic analysis reveals global-scale patterns of ocean nutrient limitation. Science 372, 287–291 (2021).
Weber, T. & Deutsch, C. Local versus basin-scale limitation of marine nitrogen fixation. Proc. Natl Acad. Sci. USA 111, 8741–8746 (2014).
Wang, W.-L., Moore, J. K., Martiny, A. C. & Primeau, F. W. Convergent estimates of marine nitrogen fixation. Nature 566, 205–211 (2019).
Repeta, D. J. et al. Marine methane paradox explained by bacterial degradation of dissolved organic matter. Nat. Geosci. 9, 884–887 (2016).
Schlitzer, R. Ocean Data View (2021); https://odv.awi.de
Monaghan, E. J. & Ruttenberg, K. C. Dissolved organic phosphorus in the coastal ocean: reassessment of available methods and seasonal phosphorus profiles from the Eel River Shelf. Limnol. Oceanogr. 44, 1702–1714 (1999).
Armstrong, Fa. J., Williams, P. M. & Strickland, J. D. H. Photo-oxidation of organic matter in sea water by ultra-violet radiation, analytical and other applications. Nature 211, 481–483 (1966).
Pujo-Pay, M. & Raimbault, P. Improvement of the wet-oxidation procedure for simultaneous determination of particulate organic nitrogen and phosphorus collected on filters. Mar. Ecol. Prog. Ser. 105, 203–207 (1994).
NASA Goddard Space Flight Center, Ocean Ecology Laboratory & Ocean Biology Processing Group Moderate-Resolution Imaging Spectroradiometer (MODIS) Aqua Chlorophyll a 9 km Data (NASA OB.DAAC, accessed 20 Jan 2021); https://oceancolor.gsfc.nasa.gov/l3/
Vapnik, V. The Nature of Statistical Learning Theory 2nd edn (Springer, 2013)
Breiman, L. Random forests. Mach. Learn. 45, 5–32 (2001).
Rasmussen, C. E. & Williams, C. K. I. Gaussian Processes for Machine Learning (MIT Press, 2005).
Trujillo-Ortiz, A. & Hernandez-Walls, R. gmregress: Geometric Mean Regression (Reduced Major Axis Regression) (MathWorks, 2022); https://www.mathworks.com/matlabcentral/fileexchange/27918-gmregress
Pawlowicz, R. M_Map: A Mapping Package for MATLAB v.1.4m (UBC EOAS, 2020); https://www.eoas.ubc.ca/~rich/map.html
Knapp, A. N., Liang, Z. & Letscher, R. T. DOP Concentration Observations From the Global Ocean Between 1990 and 2021 (DOP N2 Fixation and Export Production Project) Version 2 (Biological and Chemical Oceanography Data Management Office, 2022); https://doi.org/10.26008/1912/bco-dmo.855139.2
Acknowledgements
The work was supported by NSF OCE-1829797 (A.N.K.) and NSF OCE-1829916 (R.T.L.). We also gratefully acknowledge T.K. Westberry who provided useful feedback on NPQ-corrected φsat.
Author information
Authors and Affiliations
Contributions
Z.L. performed the analysis. Z.L. and A.N.K. designed the study. Z.L., A.N.K. and R.T.L. wrote the paper. A.N.K. and R.T.L. led the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Geoscience thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editors: James Super and Kyle Frischkorn, in collaboration with the Nature Geoscience team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Boxplots of mean DOP concentrations in the surface ocean (0–50 m) in different ocean basins.
Asterisks denote confidence levels when testing for unique mean concentrations between basins with ****: P < 0.0001, ***: P < 0.001, *: P < 0.05, using Dunn test (pairwise Kruskal–Wallis test) with Bonferroni correction. Black dots above the Eastern North Pacific and Gulf of Mexico are outliers. Center line is median and box limits are upper and lower quartiles. Whiskers show 1.5x interquartile range.
Extended Data Fig. 2 Correlation between observed upper 50 m DOP stock (mmol m−2) and mean upper 50 m P* (µM) computed from the same samples.
Black solid line is the best fit line using a Type II linear regression model and dashed blue lines are the 95% confidence level. Three stations from the BIOSOPE cruise had only phosphate but no nitrate concentration measurements and they are not included in this figure.
Extended Data Fig. 3 Correlation between observed upper 50 m DOP stock (mmol m−2) and climatological mean P* (µM) between 100 m and 250 m computed from the World Ocean Atlas 2013 (ref. 24).
Black solid line is the best fit line using a Type II linear regression model and dashed blue lines are the 95% confidence level. There are 12 stations containing DOP data with a bottom depth <100 m which have not been included in this figure.
Extended Data Fig. 4 Annual mean surface geostrophic currents (0.25° × 0.25°) and identified surface current convergence zones (SCZ).
Annual mean geostrophic currents (0.25° × 0.25°) are obtained from the Copernicus Marine Environmental Monitoring Service (marine.copernicus.eu). Three surface current convergence zones (SCZ) are identified in the North Pacific, South Pacific and South Atlantic by red circle.
Extended Data Fig. 5
Predicted surface ocean DOP concentrations (µM) based on the linear relationship between DOP concentrations and P* (Fig. 1; see main text).
Supplementary information
Supplementary Information
Supplementary Figs. 1 and 2.
Rights and permissions
About this article
Cite this article
Liang, Z., Letscher, R.T. & Knapp, A.N. Dissolved organic phosphorus concentrations in the surface ocean controlled by both phosphate and iron stress. Nat. Geosci. 15, 651–657 (2022). https://doi.org/10.1038/s41561-022-00988-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41561-022-00988-1
This article is cited by
-
Atmospheric deposition and river runoff stimulate the utilization of dissolved organic phosphorus in coastal seas
Nature Communications (2024)
-
Methylphosphonate-driven methane formation and its link to primary production in the oligotrophic North Atlantic
Nature Communications (2023)
-
A global ocean dissolved organic phosphorus concentration database (DOPv2021)
Scientific Data (2022)
-
Global patterns and predictors of C:N:P in marine ecosystems
Communications Earth & Environment (2022)