Abstract
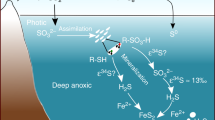
Oceanic sulfate concentrations are widely thought to have reached millimolar levels during the Proterozoic Eon, 2.5 to 0.54 billion years ago. Yet the magnitude of the increase in seawater sulfate concentrations over the course of the Eon remains largely unquantified. A rise in seawater sulfate concentrations has been inferred from the increased range of marine sulfide δ34S values following the Great Oxidation Event and was induced by two processes: enhanced oxidative weathering of sulfides on land, and the onset of marine sulfur redox cycling. Here we use mass balance and diagenetic reaction-transport models to reconstruct the sulfate concentrations in Proterozoic seawater. We find that sulfate concentrations remained below 400 µM, and were possibly as low as 100 µM, throughout much of the Proterozoic. At these low sulfate concentrations, relatively large sulfate–pyrite sulfur isotope differences cannot be explained by sulfate reduction alone and are only possible through oxidative sediment sulfur cycling. This requires oxygen concentrations of at least 10 µM in shallow Proterozoic seawater, which translates to 1–10% of present atmospheric oxygen concentrations. At these oxygen and sulfate concentrations, the oceans would have been a substantial source of methane to the atmosphere (60–140 Tmol yr−1). This methane would have accumulated to high concentrations (more than 25 ppmv) and supported greenhouse warming during much of the Proterozoic Eon, with notable exceptions during the Palaeoproterozoic and Neoproterozoic eras.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The authors declare that data supporting the findings of this study are available within this article and its Supplementary Information, and all additional data are available from the corresponding author on request.
Code availability
All additional computer codes are available from the corresponding author on request.
References
Jørgensen, B. B. Mineralization of organic matter in the sea bed—the role of sulphate reduction. Nature 296, 643–645 (1982).
Holser, W. T. & Kaplan, I. R. Isotope geochemistry of sedimentary sulfates. Chem. Geol. 1, 93–135 (1966).
Knittel, K. & Boetius, A. Anaerobic oxidation of methane: progress with an unknown process. Annu. Rev. Microbiol. 63, 311–334 (2009).
Habicht, K. S. et al. Calibration of sulfate levels in the Archean ocean. Science 298, 2372–2374 (2002).
Crowe, S. A. et al. Sulfate was a trace constituent of Archean seawater. Science 346, 735–739 (2014).
Fakhraee, M. et al. Sedimentary sulfur isotopes and Neoarchean ocean oxygenation. Sci. Adv. 4, e1701835 (2018).
Kasting, J. F. Methane and climate during the Precambrian era. Precambr. Res. 137, 119–129 (2005).
Cameron, E. M. Sulphate and sulphate reduction in early Precambrian oceans. Nature 296, 145–148 (1982).
Canfield, D. E. & Farquhar, J. Animal evolution, bioturbation, and the sulfate concentration of the oceans. Proc. Natl Acad. Sci. USA 106, 8123–8127 (2009).
Williford, K. H. et al. Constraining atmospheric oxygen and seawater sulfate concentrations during Paleoproterozoic glaciation: in situ sulfur three-isotope microanalysis of pyrite from the Turee Creek Group, Western Australia. Geochim. Cosmochim. Acta. 75, 5686–5705 (2011).
Philippot, P. et al. Globally asynchronous sulphur isotope signals require re-definition of the Great Oxidation Event. Nat. Commun. 9, 2245 (2018).
Hoffman, P. F. et al. A Neoproterozoic snowball earth. Science 281, 1342–1346 (1998).
Olson, S. L. et al. Limited role for methane in the mid-Proterozoic greenhouse. Proc. Natl Acad. Sci. USA 113, 11447–11452 (2016).
Kah, L. C., Timothy, W. L. & Frank, T. D. Low marine sulphate and protracted oxygenation of the Proterozoic biosphere. Nature 431, 834 (2004).
Canfield, D. E. A new model for Proterozoic ocean chemistry. Nature 396, 450–453 (1998).
Canfield, D. E. et al. High isotope fractionations during sulfate reduction in a low-sulfate euxinic ocean analog. Geology 38, 415–418 (2010).
Sim, M. S. et al. Large sulfur isotope fractionation does not require disproportionation. Science 333, 74–77 (2011).
Planavsky, N. J. et al. Widespread iron-rich conditions in the mid-Proterozoic ocean. Nature 477, 448–451 (2011).
Michiels, C. C. et al. Iron-dependent nitrogen cycling in a ferruginous lake and the nutrient status of Proterozoic oceans. Nat. Geosci. 3, 217–221 (2017).
Planavsky, N. J. et al. The evolution of the marine phosphate reservoir. Nature 467, 1088–1090 (2010).
Watanabe, Y. et al. Carbon, nitrogen, and sulfur geochemistry of Archean and Proterozoic shales from the Kaapvaal Craton, South Africa. Geochim. Cosmochim. Acta 61, 3441–3459 (1997).
Canfield, D. E. & Thamdrup, B. The production of 34S-depleted sulfide during bacterial disproportionation of elemental sulfur. Science 266, 1973–1975 (1994).
Johnston, D. T. et al. Active microbial sulfur disproportionation in the Mesoproterozoic. Science 310, 1477–1479 (2005).
Zhang, S. et al. Sufficient oxygen for animal respiration 1,400 million years ago. Proc. Natl Acad. Sci. USA 113, 1731–1736 (2016).
Tostevin, R. et al. Low-oxygen waters limited habitable space for early animals. Nat. Commun. 7, 12818 (2016).
Stolper, D. A. & Keller, C. B. A record of deep-ocean dissolved O2 from the oxidation state of iron in submarine basalts. Nature 553, 323–327 (2018).
Devol, A. H. & Hartnett, H. E. Role of the oxygen‐deficient zone in transfer of organic carbon to the deep ocean. Limnol. Oceanogr. 46, 1684–1690 (2001).
Andersson, J. H. et al. Respiration patterns in the deep ocean. Geophys. Res. Lett. GL018756 (2004).
Luo, G. et al. Decline in oceanic sulfate levels during the early Mesoproterozoic. Precambr Res. 258, 36–47 (2015).
Wang, X. et al. Oxygen, climate and the chemical evolution of a 1400 million year old tropical marine setting. Am. J. Sci. 317, 861–900 (2017).
Hardisty, D. F. et al. Perspectives on Proterozoic surface ocean redox from iodine contents in ancient and recent carbonate. Earth Planet. Sci. Lett. 463, 159–170 (2017).
Zhang, S. et al. The oxic degradation of sedimentary organic matter 1400 Ma constrains atmospheric oxygen levels. Biogeosciences 14, 2133–2149 (2017).
Laakso, T. A. & Schrag, D. P. A small marine biosphere in the Proterozoic. Geobiology 17, 161–171 (2018).
Kah, L. C. et al. Geochemistry of a 1.2 Ga carbonate-evaporite succession, northern Baffin and Bylot Islands: implications for Mesoproterozoic marine evolution. Precambr. Res. 111, 203–234 (2001).
Planavsky, N. J. et al. Sulfur record of rising and falling marine oxygen and sulfate levels during the Lomagundi event. Proc. Natl Acad. Sci. USA 109, 18300–18305 (2012).
Blättler, C. L. et al. Two-billion-year-old evaporites capture Earth’s great oxidation. Science 360, 320–323 (2018).
Sahoo, S. K. et al. Ocean oxygenation in the wake of the Marinoan glaciation. Nature 489, 546–549 (2012).
Lyons, T. W. et al. Tracking euxinia in the ancient ocean: a multiproxy perspective and Proterozoic case study. Annu. Rev. Earth Planet. Sci. 37, 507–534 (2009).
Fakhraee, M. et al. Significant role of organic sulfur in supporting sedimentary sulfate reduction in low-sulfate environments. Geochim. Cosmochim. Acta 213, 502–516 (2017).
Poulton, S. W. & Canfield, D. E. Ferruginous conditions: a dominant feature of the ocean through Earth’s history. Elements 7, 107–112 (2011).
Katsev, S. & Dittrich, M. Modeling of decadal scale phosphorus retention in lake sediment under varying redox conditions. Ecol. Model. 251, 246–259 (2013).
Poavlov, A. A., Hurtgen, M. T., Kasting, J. F. & Arthur, M. A. Methane-rich proterozoic atmosphere. Geology 31, 87–90 (2003).
Reeburgh, W. S. Methane consumption in Cariaco Trench waters and sediments. Earth Planet. Sci. Lett. 28, 337–344 (1976).
Laakso, T. A. & Schrag, D. P. A theory of atmospheric oxygen. Geobiology 15, 366–384 (2017).
Beal, E. J., Claire, M. W. & House, C. H. High rates of anaerobic methanotrophy at low sulfate concentrations with implications for past and present methane levels. Geobiology 9, 131–139 (2011).
Van Bodegom, P., Stams, F., Mollema, L., Boeke, S. & Leffelaar, P. Methane oxidation and the competition for oxygen in the rice rhizosphere. Appl. Environ. Microbiol. 67, 3586–3597 (2001).
Lopes, F. et al. Biogeochemical modelling of anaerobic vs. aerobic methane oxidation in a meromictic crater lake (Lake Pavin, France). Appl. Geochem. 26, 1919–1932 (2011).
Reinhard, C. T. et al. Proc. Natl Acad. Sci. USA 113, 8933–8938 (2016).
Zhao, M., Reinhard, C. T. & Planavsky, N. Terrestrial methane fluxes and Proterozoic climate. Geology 46, 139–142 (2017).
Fiorella, R. P. & Sheldon, N. D. Equable end Mesoproterozoic climate in the absence of high CO2. Geology 45, 231–234 (2017).
Bekker, A. in Encyclopedia of Astrobiology (eds Amils, R. et al.) 1–6 (2014).
Karhu, J. A. & Holland, H. D. Carbon isotopes and the rise of atmospheric oxygen. Geology 24, 867–870 (1996).
Bachan, A. & Kump, L. R. The rise of oxygen and siderite oxidation during the Lomagundi Event. Proc. Natl Acad. Sci. USA 112, 6562–6567 (2015).
Canfield, D. E. et al. A Mesoproterozoic iron formation. Proc. Natl Acad. Sci. USA 115, E3895–E3904 (2018).
Fakhraee, M. A New Insight into the Geochemistry of Sulfur in Low Sulfate Environments (2018).
Canfield, D. E. Sulfate reduction and oxic respiration in marine sediments: implications for organic carbon preservation in euxinic environments. Deep Sea Res. Pt A 36, 121–138 (1989).
Acknowledgements
This work was supported in part through an Agouron Institute fellowship to S.A.C. and NSERC discovery grant no. 0487 to S.A.C. This study was also supported by the Danish National Research Foundation (grant no. DNRF53) to D.E.C.
Author information
Authors and Affiliations
Contributions
M.F., S.K. and S.A.C. designed the research. M.F. and S.K. developed the diagenetic model. S.A.C. and D.E.C. developed the mass balance model with input from O.H. M.F. performed mass balance and diagenetic model simulations and sensitivity analyses. M.F., S.K. and S.A.C. interpreted model results and wrote the paper, with contributions from D.E.C.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary Information
Supplementary modelling information, Supplementary Figs. 1–14 and Supplementary Tables 1–7.
Rights and permissions
About this article
Cite this article
Fakhraee, M., Hancisse, O., Canfield, D.E. et al. Proterozoic seawater sulfate scarcity and the evolution of ocean–atmosphere chemistry. Nat. Geosci. 12, 375–380 (2019). https://doi.org/10.1038/s41561-019-0351-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41561-019-0351-5
This article is cited by
-
A phosphate-rich marine reservoir in the redox stratified Ediacaran ocean
Communications Earth & Environment (2024)
-
Dynamic redox and nutrient cycling response to climate forcing in the Mesoproterozoic ocean
Nature Communications (2023)
-
Influence of sulfide on diazotrophic growth of the methanogen Methanococcus maripaludis and its implications for the origin of nitrogenase
Communications Biology (2023)
-
Late acquisition of the rTCA carbon fixation pathway by Chlorobi
Nature Ecology & Evolution (2023)
-
Oxidized sulfur-rich arc magmas formed porphyry Cu deposits by 1.88 Ga
Nature Communications (2021)