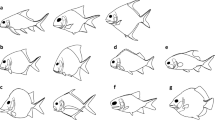
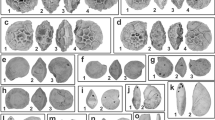
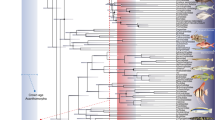
Abstract
Many accounts of the early history of actinopterygians (ray-finned fishes) posit that the end-Devonian mass extinction had a major influence on their evolution. Existing phylogenies suggest this episode could have acted as a bottleneck, paring the early diversity of the group to a handful of survivors. This picture, coupled with increases in taxonomic and morphological diversity in the Carboniferous, contributes to a model of explosive post-extinction radiation. However, most actinopterygians from within a roughly 20-million year (Myr) window surrounding the extinction are poorly known, contributing to uncertainty about the meaning of these patterns. Here, we report an exceptionally preserved fossil from 7 Myr before the extinction that reveals unexpected anatomical features. Palaeoneiros clackorum gen. et sp. nov. nests within a clade of post-Devonian species and, in an expanded phylogenetic analysis, draws multiple lineages of Carboniferous actinopterygians into the Devonian. This suggests cryptic but extensive lineage diversification in the latest Devonian, followed by more conspicuous feeding and locomotor structure diversification in the Carboniferous. Our revised model matches more complex patterns of divergence, survival and diversification around the Devonian/Carboniferous boundary in other vertebrate clades. It also fundamentally recalibrates the onset of diversification early in the history of this major radiation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The specimen described in this study is deposited in the collections of the Harvard Museum of Comparative Zoology. CT data and derivatives are available on Morphosource (projection series: https://doi.org/10.17602/M2/M417031; TIFF stack: https://doi.org/10.17602/M2/M420055; OBJ file of all segmented three-dimensional objects https://doi.org/10.17602/M2/M420865; PLY file of all segemented three-dimensional objects: https://doi.org/10.17602/M2/M420629). The Mimics file can be downloaded by contacting the Department of Vertebrate Paleontology at the Museum of Comparative Zoology, Harvard University. All other data associated with this paper are included in the Supplementary Data. This work and associated nomenclatural acts are registered in ZooBank. ZooBank LSIDs are accessible at the URL generated by appending the LSID to http://zoobank.org.
Code availability
The code needed to perform the trait analyses is included in the Supplementary Data.
References
Woodward, A. S. Catalogue of the Fossil Fishes in the British Museum (Natural History). Part II. Containing the Elasmobranchii (Acanthodii), Holocephali, Ichthyodorulites, Ostracodermi, Dipnoi, and Teleostomi (Crossopterygii and Chondrostean Actinopterygii) (British Museum, 1881).
Woodward, A. S. Catalogue of the Fossil Fishes in the British Museum (Natural History). Part III. Containing the Actinopterygian Teleostomi of the Orders Chondrostei (Concluded), Protospondyli, Aethospondyli, and Isospondyli (In Part) (British Museum, 1895).
Sallan, L. C. & Coates, M. I. End-Devonian extinction and a bottleneck in the early evolution of modern jawed vertebrates. Proc. Natl Acad. Sci. USA 107, 10131–10135 (2010).
Smithson, T. R., Richards, K. R. & Clack, J. A. Lungfish diversity in Romer’s Gap: reaction to the end-Devonian extinction. Palaeontology 59, 29–44 (2016).
Anderson, J. S., Smithson, T., Masksy, C. F., Meyer, T. & Clack, J. A diverse tetrapod fauna from the base of ‘Romer’s Gap’. PLoS ONE 10, e0125446 (2015).
Smithson, T. R., Wood, S. P., Marshall, J. E. A. & Clack, J. A. Earliest Carboniferous tetrapod and arthropod faunas populate Romer’s Gap. Proc. Natl Acad. Sci. USA 109, 4532–4537 (2012).
Long, J. A. & Trinajstic, K. The Late Devonian Gogo Formation Lägerstatte of Western Australia: exceptional early vertebrate preservation and diversity. Annu. Rev. Earth Planet. Sci. 38, 255–279 (2010).
Trewin, N. H. Palaeoecology and sedimentology of the Achanarras fish bed of the Middle Old Red Sandstone, Scotland. Trans. R. Soc. Edinb. Earth Sci. 77, 21–46 (1986).
Sallan, L. C. & Friedman, M. Heads or tails: staged diversification in vertebrate evolutionary radiations. Proc. Biol. Sci. 279, 2025–2032 (2012).
Friedman, M., Pierce, S. E., Coates, M. & Giles, S. Feeding structures in the ray-finned fish Eurynotus crenatus (Actinopterygii: Eurynotiformes): implications for trophic diversification among Carboniferous actinopterygians. Earth Environ. Sci. Trans. R. Soc. Edinb. 109, 33–47 (2018).
Sallan, L. C. & Coates, M. I. Styracopterid (Actinopterygii) ontogeny and the multiple origins of post-Hangenberg deep-bodied fishes. Zool. J. Linn. Soc. 169, 156–199 (2013).
Sallan, L. C. Tetrapod-like axial regionalization in an early ray-finned fish. Proc. Biol. Sci. 279, 3264–3271 (2012).
Sallan, L. & Galimberti, A. K. Body-size reduction in vertebrates following the end-Devonian mass extinction. Science 350, 812–815 (2015).
Gardiner, B. G. & Schaeffer, B. Interrelationships of lower actinopterygian fishes. Zool. J. Linn. Soc. 97, 135–187 (1989).
Coates, M. I. Endocranial preservation of a Carboniferous actinopterygian from Lancashire, UK, and the interrelationships of primitive actinopterygians. Philos. Trans. R. Soc. Lond. B 354, 435–462 (1999).
Giles, S., Xu, G.-H., Near, T. J. & Friedman, M. Early members of a ‘living fossil’ lineage imply later origin of modern ray-finned fishes. Nature 549, 265–268 (2017).
Cloutier, R. & Arraita, G. in Recent Advances in the Origin and Early Radiation of Vertebrates (eds Arratia, G. et al.) 217–270 (Dr. Friedrich Pfeil Verlag, 2004).
O’Leary, M. A. et al. The placental mammal ancestor and the post-K-Pg radiation of placentals. Science 339, 662–667 (2013).
Wilson, C. D., Pardo, J. D. & Anderson, J. S. A primitive actinopterygian braincase from the Tournaisian of Nova Scotia. R. Soc. Open Sci. 5, 171727 (2018).
Gardiner, B. G. The relationships of the palaeoniscid fishes, a review based on new species of Mimia and Moythomasia from the Upper Devonian of Western Australia. Bull. Br. Mus. Geol. 37, 173–428 (1984).
Coates, M. I. Actinoptergians from the Namurian of Bearsden, Scotland, with comments on early actinopterygian neurocrania. Zool. J. Linn. Soc. 122, 27–59 (1998).
Poplin, C. Étude de Quelques Paléoniscidés Pennsylvaniens du Kansas (Éditions du CNRS, 1974).
Dunkle, D. H. Preliminary Description of a Paleoniscoid Fish from the Upper Devonian of Ohio (Cleveland Museum of Natural History, 1964).
Friedman, M. & Blom, H. A new actinopterygian from the Famennian of East Greenland and the interrelationships of Devonian ray-finned fishes. J. Paleontol. 80, 1186–1204 (2006).
Daeschler, E. B. An early actinopterygian fish from the Catskill Formation (Late Devonian, Famennian) in Pennsylvania, U.S.A. Proc. Acad. Nat. Sci. Phila. 150, 181–192 (2000).
Prokofiev, A. M. First finding of an articulated actinopterygian skeleton from the Upper Devonian of Siberia and a reappraisal of the family Moythomasiidae Kazantseva, 1971 (Osteichthyes). Paleontol. Res. 6, 321–327 (2002).
Janvier, P., Lethiers, F., Monod, O. & Balkaş, Ö. Discovery of a vertebrate fauna at the Devonian-Carboniferous boundary in SE Turkey (Hakkari Province). J. Pet. Geol. 7, 147–168 (1984).
Wilson, C. D., Mansky, C. F. & Anderson, J. S. A platysomid occurrence from the Tournaisian of Nova Scotia. Sci. Rep. 11, 8375 (2021).
Eastman, C. R. Devonic Fishes of the New York Formations (New York State Education Department, 1907).
Challands, T. J. et al. A lungfish survivor of the end-Devonian extinction and an early Carboniferous dipnoan radiation. J. Syst. Palaeontol. 17, 1825–1846 (2019).
Clack, J. A., Challands, T. J., Smithson, T. R. & Smithson, K. Z. Newly recognized Famennian lungfishes from East Greenland reveal tooth plate diversity and blur the Devonian-Carboniferous boundary. Pap. Palaeontol. 5, 261–279 (2019).
Anderson, J. S., Smithson, T., Mansky, C. F., Meyer, T. & Clack, J. A diverse tetrapod fauna at the base of ‘Romer’s Gap’. PLoS ONE 10, e102446 (2015).
Daeschler, E. B., Clack, J. A. & Shubin, N. H. Late Devonian tetrapod remains from Red Hill, Pennsylvania, USA: how much diversity? Acta Zool. 90, 306–317 (2009).
Ahlberg, P. E. & Clack, J. A. The smallest known Devonian tetrapod shows unexpectedly derived features. R. Soc. Open Sci. 7, 192117 (2020).
Pardo, J. D., Szostakiwskyj, M., Ahlberg, P. E. & Anderson, J. S. Hidden morphological diversity among early tetrapods. Nature 546, 642–645 (2017).
Socolow, A. A. (ed) Geologic Map of Pennsylvania, 1:250,000(Commonwealth of Pennsylvania, Department of Environmental Resources, 1980).
Startenaer, P. Jacoburbirostrum, a new middle Famennian rhynchonellid (brachiopod) genus from southwestern New York State. Bull. Geosci. 89, 607–616 (2014).
Kirchgasser, W. T. in Recognition of Devonian Series and Stage Boundaries in Geological Areas. Vol. 225. Courier Forschungsinstitut Senckenberg (ed. Bultynck, P.) 271–284 (2000).
House, M. R. & Kirchgasser, W. Late Devonian goniatites (Cephalopoda, Ammonoidea) from New York State. Bull. Am. Paleontol. 374, 1–294 (2008).
Clendening, J. A., Eames, L. E. & Wood, G. D. Retusotriletes phillipsii n. sp., a potential Upper Devonian guide palynomorph. Palynology 4, 15–21 (1980).
Becker, R. T., Marshall, J. E. A. & Da Silva, A.-C. in Geologic Time Scale 2020 (eds Gradstein, F. M. et al.) 733–810 (Elsevier, 2020).
Yeager, K. M. Fossil fishes (Arthrodira and Acanthodida) from the Upper Devonian Chadokoin Formation of Erie County, Pennsylvania. Ohio J. Sci. 96, 52–56 (1996).
Lowney, K. A. Certain Bear Gulch (Namurian A, Montana) Actinopterygii (Osteichthyes), and a Re-evaluation of the Evolution of the Paleozoic Actinopterygians. PhD thesis, New York Univ. (1980).
Coates, M. I. New actinopterygian fish from the Namurian Manse Burn Formation of Bearsden, Scotland. Palaeontology 36, 123–146 (1993).
Schultze, H.-P. & Bardack, D. Diversity and size changes in palaeonisciform fishes (Actinopterygii, Pisces) from the Pennsylvanian Mazon Creek fauna, Illinois, U.S.A. J. Vertebr. Paleontol. 7, 1–23 (1987).
Rayner, D. H. On the cranial structure of an early palæoniscid, Kentuckia, gen. nov. Trans. R. Soc. Edinb. 62, 53–83 (1952).
Hamel, M. H. & Poplin, C. The braincase anatomy of Lawrenciella schaefferi, actinopterygian from the Upper Carboniferous of Kansas (USA). J. Vertebr. Paleontol. 28, 989–1006 (2008).
Schaeffer, B. & Dalquest, W. W. A palaeonisciform braincase from the Permian of Texas, with comments on cranial fissures and the posterior myodome. Am. Mus. Novit. 2658, 1–15 (1978).
Nielsen, E. Studies on Triassic fishes from East Greenland. I. Glaucolepis and Boreosomus. Meddr. Grønland 138, 1–403.
Jessen, H. Moythomasia nitida Gross und M. cf. striata Gross, Devonische Palaeonisciden aus dem oberen Plattenkalk der Bergisch-Gladbach-Paffrather Mulde (Rheinisches Schiefergebirge) [in German]. Palaeontogr. Abt. A 128, 87–114 (1968).
Choo, B. A new species of the Devonian actinopterygian Moythomasia from Bergisch Gladbach, Germany, and fresh observations on M. durgaringa from the Gogo Formation of Western Australia. J. Vertebr. Paleontol. 35, e952817 (2015).
Taverne, L. P. Osorioichthys marginis, “paléonisciforme” du Famennien de Belgique, et la phylogénie des Actinoptérygiens dévoniens (Pisces). Bull. de l’Institut R. des Sci. Naturelles de Belgique 67, 57–78 (1997).
Moy-Thomas, J. A. Memoirs: notes on the development of the chondrocranium in Polypterus senegalus. J. Cell Sci. 76, 209–229 (1933).
Daget, J., Bauchot, M. L., Bauchot, R. & Arnoult, J. Développement du chondrocrâne et des arcs aortiques chez Polypterus senegalus Cuvier. Acta Zool. 45, 201–244 (1964).
Latimer, A. E. & Giles, S. A giant dapediid from the Late Triassic of Switzerland and insights into neopterygian phylogeny. R. Soc. Open Sci. 5, 180497 (2018).
Gardiner, B. G., Schaeffer, B. & Masserie, J. A. A review of the lower actinopterygian phylogeny. Zool. J. Linn. Soc. 144, 511–525 (2005).
Mickle, K. E. & Lund, R. Three new palaeoniscoid fishes from the Bear Gulch Limestone (Serpukhovian, Mississippian) of Montana (USA) and the relationships of lower actinopterygians. Geodiversitas 31, 623–668 (2009).
Sallan, L. C. Major issues in the origin of ray-finned fish (Actinopterygii) biodiversity. Biol. Rev. Camb. Philos. Soc. 89, 950–971 (2014).
Friedman, M. The early evolution of ray-finned fishes. Palaeontology 58, 213–228 (2015).
Henderson, S., Dunne, E. M., Fasey, S. & Giles, S. The early diversification of ray-finned fishes (Actinopterygii): hypotheses, challenges, and future prospects. Biol. Rev. (2022).
Bapst, D. W. & Hopkins, M. J. Comparing cal3 and other a posteriori time-scaling approaches in a case study with the pterocephaliid trilobites. Paleobiology 43, 49–67 (2017).
Heath, T. A., Huelsenbeck, J. P. & Stadler, T. The fossilized birth–death process for coherent calibration of divergence–time estimates. Proc. Natl Acad. Sci. USA 111, E2957–E2966 (2014).
Monarrez, P. M., Heim, N. A. & Payne, J. L. Mass extinctions alter extinction and origination dynamics with respect to body size. Proc. Biol. Sci. 288, 20211681 (2021).
Cantalapiedra, J. L., Prado, J. L., Hernández Fernández, M. & Alberdi, M. T. Decoupled ecomorphological evolution and diversification in Neogene-Quaternary horses. Science 355, 627–630 (2017).
Simões, T., Vernygora, O., Caldwell, M. W. & Pierce, S. E. Megaevolutionary dynamics and the timing of evolutionary innovation in reptiles. Nat. Commun. 11, 3322 (2020).
Upham, N. S., Esselstyn, J. A. & Jetz, W. Inferring the mammal tree: species-level sets of phylogenies for questions in ecology, evolution, and conservation. PLoS Biol. 17, e3000494 (2019).
Alroy, J. The fossil record of North American mammals: evidence for a Paleocene evolutionary radiation. Syst. Biol. 48, 107–118 (1999).
Goloboff, P. A., Farris, J. S. & Nixon, K. C. TNT, a free program for phylogenetic analysis. Cladistics 24, 774–786 (2008).
Ronquist, F. et al. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 61, 539–542 (2012).
Figueroa, R. T., Weinschütz, L. C. & Friedman, M. The oldest Devonian circumpolar ray-finned fish? Biol. Lett. 17, 20200766 (2021).
Swofford, D. L. PAUP* [*Phylogenetic Analysis Using Parsimony (and Other Methods)] 4,0 b (Sinauer Associates, 2003).
Matzke, N. J. & Wright, A. Inferring node dates from tip dates in fossil Canidae: the importance of tree priors. Biol. Lett. 12, 20160328 (2016).
Revell, L. J. Two new graphical methods for mapping trait evolution on phylogenies. Methods Ecol. Evol. 4, 754–759 (2013).
Revell, L. J. phytools: an R package for phylogenetic comparative biology (and other things). Methods Ecol. Evol. 3, 217–223 (2012).
Felsenstein, J. Phylogenies and the comparative method. Am. Nat. 125, 1–15 (1985).
Gardiner, B. G. Certain palaeoniscoid fishes and the evolution of the snout in actinopterygians. Bull. Br. Mus. Geol. 8, 255–325 (1963).
Acknowledgements
We thank the Museum of Comparative Zoology, Harvard University for specimen access and especially J. Cundiff for organizing the loan of material that formed the basis of this study. We also thank E. Bernard, D. Gelsthorpe, Z. Johanson, L. Loughtman, P. Shephard and S. Walsh for facilitating access to comparative collections; G. Lloyd for helpful discussions; and C. Byrd for assistance with data archiving. S.G. was supported by a Royal Society Dorothy Hodgkin Research Fellowship (no. DH160098). This research was also supported by funding from the National Science Foundation (NSF) to M.F. (NSF-GEO 2219007) and S.E.P. (NSF-GEO 2219069). This study includes data produced in the CTEES facility at University of Michigan, supported by the Department of Earth and Environmental Sciences and College of Literature, Science, and the Arts.
Author information
Authors and Affiliations
Contributions
M.F. and S.E.P. designed the research. S.G., M.F., K.F. and R.W. performed the research and analysed the data. S.G. and M.F. wrote the paper and designed figures. All authors edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Ecology & Evolution thanks Jason Pardo, Michael Gottfried and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Geological context of Palaeoneiros clackorum MCZ VPF-5114.
(A) Map of Pennsylvania showing location; inset shows geological map for the area of Warren County (based on (36)) and simplified geological column (based on (68)). City of Warren indicated by solid bounding lines. (B) External photograph of Palaeoneiros clackorum showing right lateral flank. Colors in stratigraphic column relate to geological map: latest Devonian and post-Devonian strata condensed into one color. Scale bar in (A): 10 km, in (B): 10 mm. Credit: Museum of Comparative Zoology, Harvard University; © President and Fellows of Harvard College.
Extended Data Fig. 2 External photographs of Palaeoneiros clackorum MCZ VPF-5114.
Right lateral flank in (A) part and (B) counterpart. (C) Ventral view showing entire specimen, and (D) closeup of skull and pectoral region. (E) Right anal fin. (F) Scales from right lateral flank. Scale bar in (A)-(C): 10 mm, in (D)-(F): 5 mm. Photos copyright President and Fellows of Harvard College. Abbreviations: an, anal fin; br, branchiostegal; chy, ceratohyal; clth, cleithrum; dent, dentary; dors, dorsal fin; frf, fringing fulcra; lep, lepidotrichia; mx, maxilla; pect, pectoral fin; pelv, pelvic fin; sc, scale. Credit: Museum of Comparative Zoology, Harvard University; © President and Fellows of Harvard College.
Extended Data Fig. 3 Anatomy of Palaeoneiros clackorum MCZ VPF-5114.
Interpretive drawing of (A) right lateral view of specimen; (B) medial view of right upper and lower jaws; (C) medial view of left shoulder girdle and fin, and (D) left lateral view of gill skeleton, hyoid arch and braincase. Scale bar in (A): 10 mm, in (B)-(D): 5 mm. Abbreviations: ang, angular; art, articular; asp, ascending process of parasphenoid; av, accessory vomer; br, branchiostegal; cb, ceratobranchial; chy, ceratohyal; clav, clavicle; clth, cleithrum; dent, dentary; dmpt, dermatopterygoid; dsph, dermosphenotic; ecpt, ectopterygoid and dermopalatines; eb, epibranchial; enpt, entopteryygoid; exo, exoccipital; hmd, hyomandibula;?ic, possible intercalar; icl, interclavicle; ioc, infraorbital canal; it, intertemporal; jug, jugal; jugc, jugal canal; lep, lepidotrichia; ll, lateral line; mc, mandibular canal; mx, maxilla; op, operculum; part, prearticular and coronoid; popc, preopercular canal; popl, preopercular pit lines;?pmx, possible premaxilla; psp, parasphenoid; qu, quadrate; rad, radials; sc, scale; scl, sclerotic ossicle; scpc, scapulocoracoid; sop, suboperculum; sorb, suborbitals; sph, sphenotic ossification; st, supratemporal; t, teeth; tp, toothplate; unc, uncinate process.
Extended Data Fig. 4 External anatomy of Palaeoneiros clackorum MCZ VPF-5114.
Renders of specimen in (A) right lateral, (B) left lateral, (C) dorsal, (D) anterior, and (E) ventral view; and an isolated scale (F). Colour coding of skeleton: blue, cheek and jaw; purple, skull roof and sclerotic ossicle; pink, braincase; dark green, hyomandibula; light green, operculogular system; turquoise, shoulder girdle; yellow, gill skeleton. Scale bar in (A)-(C),(E): 5 mm, in (D): 2 mm, in(F):1 mm. Abbreviations: ang, angular; antd, anterodorsal process; art, articular; asc, ascending process of the parasphenoid; av, accessory vomer; br, branchiostegal; cb, ceratobranchial; chy, ceratohyal; clav, clavicle; clth, cleithrum; dent, dentary; dsph, dermosphenotic; eb, epibranchial; ecpt, ectopterygoid and dermopalatines; enpt, entopterygoid; exsc, extrascapular; fr, frontal; fu, fused lepidotrichia; hb, hypobranchial; hmd, hyomandibula;?ic, possible intercalar; icl, interclavicle; ioc, infraorbital canal; it, intertemporal; jgl, jugal canal; jug, jugal; lac, lacrimal; lep, lepidotrichia; ll, lateral line; mc, mandibular canal; mpl, middle pit line; mx, maxilla; op, operculum; pa, parietal; part, prearticular and coronoids; peg, scale peg; pi, pineal foramen;?pmx, possible premaxilla; pop, preoperculum; popl, preopercular pit line; ppl, posterior pit line; pscl, presupracleithrum; psp, parasphenoid; pt, posttemporal; qu, quadrate; rad, radial; sc, scale; scl, sclerotic ossicle; sclth, supracleithrum; soc, supraorbital canal; sop, suboperculum; sorb, suborbital; sph, sphenotic ossification; st, supratemporal; unc, uncinate process. Renders copyright President and Fellows of Harvard College.
Extended Data Fig. 5 Renders of Palaeoneiros clackorum MCZ VPF-5114.
(A) Skull roofing bones in visceral view. (B) Possible premaxilla. (C) Right maxilla, dentary and postdentary in medial view. (D) Right lower jaw in dorsal view. (E) Left lower jaw in medial view. Scale bar in all: 2 mm. Abbreviations: add, adductor fossa; ang, angular; art, articular; dent, dentary; exsc, extrascapular; fr, frontal; ll, lateral line; mc, mandibular canal; mx, maxilla; na, nasal; ov, maxilla overlap of dentary;?pmx, premaxilla; part, prearticular plus coronoids; pi, pineal foramen; pt, posttemporal; qu, quadrate; ri, ridge; pa, parietal; sh, maxillary shelf; soc, supraorbital canal; t, tooth; tp, toothplate. Credit: Museum of Comparative Zoology, Harvard University; © President and Fellows of Harvard College.
Extended Data Fig. 6 Renders of gill skeleton of Palaeoneiros clackorum MCZ VPF-5114.
(A) Gill skeleton in dorsal view. (B) Gill skeleton in ventral view. Scale bar: 5 mm. Abbreviations: bb, basibranchial; cb, ceratobranchial; eb, epibranchial; hb, hyobranchial; pb, pharyngobranchial. Credit: Museum of Comparative Zoology, Harvard University; © President and Fellows of Harvard College.
Extended Data Fig. 7 Renders of shoulder and fin of Palaeoneiros clackorum MCZ VPF-5114.
(A) Shoulder girdle and pectoral fin in left lateral view, and (B) closeup with dermal girdle removed. (C) Shoulder girdle and pectoral fin in medial view, and (D) with radials and lepidotrichia removed. (E) Shoulder girdle and pectoral fin in posterior view, and (F) with radials and lepidotrichia removed. Radials in (G) dorsal and (H) ventral view. Scale bar in (A)-(F): 2 mm, in (G)-(H): 1 mm. Abbreviations: clav, clavicle; clth, cleithrum; fu, fused lepidotrichia; gl, glenoid fossa; icl, interclavicle; lep, lepidotrichia; mptg, metapterygium; pr.rad, preaxial radial; ptg, propterygium; ptg.ca, propterygial canal; rad, radial; scpc.hz, horizontal plate of scapulocoracoid; scpc.pl, scapulocoracoid plates. Credit: Museum of Comparative Zoology, Harvard University; © President and Fellows of Harvard College.
Extended Data Fig. 8 Results of parsimony analysis.
(A) Strict consensus of the 20,000 trees (1616 steps) for 121 taxa and 292 equally weighted characters. Digits above nodes indicate Bremer decay indices above 1. (B) Agreement subtree. New taxon indicated in bold.
Extended Data Fig. 9 Results of Bayesian analysis.
50% majority rule tree for 121 taxa and 292 equally weighted characters. Digits at nodes indicate posterior probability support. New taxon indicated in bold.
Extended Data Fig. 10 Results of fossil birth-death analysis.
Blue bars associated with nodes indicate 95% highest posterior density for age estimates. New taxon indicated in bold.
Supplementary information
Supplementary Information
Supplementary Text, Table and References.
Supplementary Data 1
Zip file containing the files needed to perform the parsimony analysis in TNT (morphological data matrix, TNT script, constraint tree) and a consensus tree file.
Supplementary Data 2
Zip file containing the morphological data matrix formatted for the Bayesian analysis and a consensus tree file.
Supplementary Data 3
Zip file containing the code required to perform the trait analyses and the Excel file containing the data for the timescaling and lower-jaw analyses. Sheet 1 contains taxon list, data source, standard length, jaw length, age data, locality and stratigraphy. NA indicates that lower-jaw length or taxon was not included in our analysis. Red, inapplicable; orange, measured from literature; green, measured from the specimen. Sheet 2 contains the taxonomic notes. Sheet 3 contains the average jaw and standard lengths for taxa in which both can be measured.
Supplementary Data 4
Zip file containing the MrBayes script for the fossil birth–death analysis and consensus file.
Supplementary Data 5
Large-format PDF of a single most parsimonious tree showing all character optimizations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Giles, S., Feilich, K., Warnock, R.C.M. et al. A Late Devonian actinopterygian suggests high lineage survivorship across the end-Devonian mass extinction. Nat Ecol Evol 7, 10–19 (2023). https://doi.org/10.1038/s41559-022-01919-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41559-022-01919-4