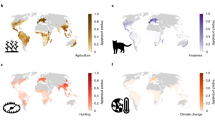
Abstract
Much of biodiversity remains undiscovered, causing species and their functions to remain unrealized and potentially lost in ignorance. Here we use extensive species-level data in a time-to-event model framework to identify taxonomic and geographic discovery gaps in terrestrial vertebrates. Biological, environmental and sociological factors all affect discovery probability and together provide strong predictive ability for species discovery. Our model identifies distinct taxonomic and geographic unevenness in future discovery potential, with greatest opportunities for amphibians and reptiles, and for Neotropical and Indo-Malayan forests. Brazil, Indonesia, Madagascar and Colombia emerge as holding greatest discovery opportunities, with a quarter of potential discoveries estimated. These findings highlight the importance of international policy support for basic taxonomic research and the potential of quantitative models to aid species discovery.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Data produced for this study are available as Supplementary Data files. Raw data to reproduce the analysis of this study are available at vertlife.org/data/discoverypotential.
Code availability
R scripts to reproduce the analysis of this study are available at vertlife.org/data/discoverypotential.
References
Costello, M. J., May, R. M. & Stork, N. E. Can we name Earth’s species before they go extinct? Science 339, 413–416 (2013).
Mora, C., Rollo, A. & Tittensor, D. P. Comment on ‘Can we name Earth’s species before they go extinct?’. Science 341, 237 (2013).
Mora, C., Tittensor, D. P., Adl, S., Simpson, A. G. B. & Worm, B. How many species are there on Earth and in the Ocean? PLoS Biol. 9, e1001127 (2011).
May, R. & Beverton, R. J. H. How many species? Phil. Trans. R. Soc. B 330, 293–304 (1990).
Scheffers, B. R., Joppa, L. N., Pimm, S. L. & Laurance, W. F. What we know and don’t know about Earth’s missing biodiversity. Trends Ecol. Evol. 27, 501–510 (2012).
Raven, P. H. & Wilson, E. O. A fifty-year plan for biodiversity surveys. Science 258, 1099–1100 (1992).
Whittaker, R. J. et al. Conservation biogeography: assessment and prospect. Divers. Distrib. 11, 3–23 (2005).
Hortal, J. et al. Seven shortfalls that beset large-scale knowledge of biodiversity. Annu. Rev. Ecol. Evol. Syst. 46, 523–549 (2015).
Guide to the Global Taxonomy Initiative (Secretariat of the Convention on Biological Diversity, 2010).
Costello, M. J., May, R. M. & Stork, N. E. Response to comments on ‘Can we name Earth’s species before they go extinct?’. Science 341, 237 (2013).
Bebber, D. P., Marriott, F. H. C., Gaston, K. J., Harris, S. A. & Scotland, R. W. Predicting unknown species numbers using discovery curves. Proc. R. Soc. B 274, 1651–1658 (2007).
Edie, S. M., Smits, P. D. & Jablonski, D. Probabilistic models of species discovery and biodiversity comparisons. Proc. Natl Acad. Sci. USA 114, 3666–3671 (2017).
Guenard, B., Weiser, M. D. & Dunn, R. R. Global models of ant diversity suggest regions where new discoveries are most likely are under disproportionate deforestation threat. Proc. Natl Acad. Sci. USA 109, 7368–7373 (2012).
Blackburn, T. M. & Gaston, K. J. What determines the probability of discovering a species - a study of South-American Oscine Passerine birds. J. Biogeogr. 22, 7–14 (1995).
Costello, M. J., Lane, M., Wilson, S. & Houlding, B. Factors influencing when species are first named and estimating global species richness. Glob. Ecol. Conserv. 4, 243–254 (2015).
Collen, B., Purvis, A. & Gittleman, J. L. Biological correlates of description date in carnivores and primates. Glob. Ecol. Biogeogr. 13, 459–467 (2004).
Diniz-Filho, J. A. F. et al. Macroecological correlates and spatial patterns of anuran description dates in the Brazilian Cerrado. Glob. Ecol. Biogeogr. 14, 469–477 (2005).
Costello, M. J., Houlding, B. & Joppa, L. N. Further evidence of more taxonomists discovering new species, and that most species have been named: response to Bebber et al. (2014). New Phytol. 202, 739–740 (2014).
Meiri, S. Small, rare and trendy: traits and biogeography of lizards described in the 21st century. J. Zool. 299, 251–261 (2016).
Klein, J. P. & Moeschberger, M. L. Survival Analysis: Techniques for Censored and Truncated Data.(Springer, 2003).
Essl, F., Rabitsch, W., Dullinger, S., Moser, D. & Milasowszky, N. How well do we know species richness in a well-known continent? Temporal patterns of endemic and widespread species descriptions in the European fauna. Glob. Ecol. Biogeogr. 22, 29–39 (2013).
Colli, G. R. et al. In the depths of obscurity: knowledge gaps and extinction risk of Brazilian worm lizards (Squamata, Amphisbaenidae). Biol. Conserv. 204, 51–62 (2016).
Burgin, C. J., Colella, J. P., Kahn, P. L. & Upham, N. S. How many species of mammals are there? J. Mammal. 99, 1–14 (2018).
Meyer, C., Kreft, H., Guralnick, R. & Jetz, W. Global priorities for an effective information basis of biodiversity distributions. Nat. Commun. 6, 8221 (2015).
Bellard, C. et al. Vulnerability of biodiversity hotspots to global change. Glob. Ecol. Biogeogr. 23, 1376–1386 (2014).
Quintero, I. & Jetz, W. Global elevational diversity and diversification of birds. Nature 555, 246–250 (2018).
Joppa, L. N., Roberts, D. L. & Pimm, S. L. How many species of flowering plants are there? Proc. R. Soc. B 278, 554–559 (2011).
Giam, X. et al. Reservoirs of richness: least disturbed tropical forests are centres of undescribed species diversity. Proc. R. Soc. B 279, 67–76 (2012).
Jetz, W. & Fine, P. V. A. Global gradients in vertebrate diversity predicted by historical area-productivity dynamics and contemporary environment. PLoS Biol. 10, e1001292 (2012).
Gouveia, S. F., Villalobos, F., Dobrovolski, R., Beltrão-Mendes, R. & Ferrari, S. F. Forest structure drives global diversity of primates. J. Anim. Ecol. 83, 1523–1530 (2014).
Oliveira, B. F. & Scheffers, B. R. Vertical stratification influences global patterns of biodiversity. Ecography 42, 249–249 (2019).
Oliveira, U. et al. The strong influence of collection bias on biodiversity knowledge shortfalls of Brazilian terrestrial biodiversity. Divers. Distrib. 22, 1232–1244 (2016).
Roll, U. et al. The global distribution of tetrapods reveals a need for targeted reptile conservation. Nat. Ecol. Evol. 1, 1677–1682 (2017).
Garnett, S. T. & Christidis, L. Taxonomy anarchy hampers conservation. Nature 546, 25–27 (2017).
Isaac, N. J. B., Mallet, J. & Mace, G. M. Taxonomic inflation: its influence on macroecology and conservation. Trends Ecol. Evol. 19, 464–469 (2004).
Bremer, K., Bremer, B., Karis, P. & Källersjö, M. Time for change in taxonomy. Nature 343, 202 (1990).
Raposo, M. A. et al. What really hampers taxonomy and conservation? A riposte to Garnett and Christidis (2017). Zootaxa 4317, 179–184 (2017).
Wake, D. B. Persistent plethodontid themes: species, phylogenies, and biogeography. Herpetologica 73, 242–251 (2017).
Tedesco, P. A. et al. Estimating how many undescribed species have gone extinct. Conserv. Biol. 28, 1360–1370 (2014).
Jetz, W., McPherson, J. M. & Guralnick, R. P. Integrating biodiversity distribution knowledge: toward a global map of life. Trends Ecol. Evol. 27, 151–159 (2012).
Jetz, W., Thomas, G. H., Joy, J. B., Hartmann, K. & Mooers, A. O. The global diversity of birds in space and time. Nature 491, 444–448 (2012).
Jetz, W. & Pyron, R. A. The interplay of past diversification and evolutionary isolation with present imperilment across the amphibian tree of life. Nat. Ecol. Evol. 2, 850–858 (2018).
Upham, N. S., Esselstyn, J. A. & Jetz, W. Inferring the mammal tree: species-level sets of phylogenies for questions in ecology, evolution, and conservation. PLoS Biol. 17, e3000494 (2019).
González-del-Pliego, P. et al. Phylogenetic and trait-based prediction of extinction risk for data-deficient amphibians. Curr. Biol. 29, 1557–1563.e3 (2019).
Moura, M. R. et al. Geographical and socioeconomic determinants of species discovery trends in a biodiversity hotspot. Biol. Conserv. 220, 237–244 (2018).
Gaston, K. J., Blackburn, T. M. & Loder, N. Which species are described first? The case of North-American butterflies. Biodivers. Conserv. 4, 119–127 (1995).
Oliveira, B. F., São-Pedro, V. A., Santos-Barrera, G., Penone, C. & Costa, G. C. AmphiBIO, a global database for amphibian ecological traits. Sci. Data 4, 170123 (2017).
Feldman, A., Sabath, N., Pyron, R. A., Mayrose, I. & Meiri, S. Body sizes and diversification rates of lizards, snakes, amphisbaenians and the tuatara. Glob. Ecol. Biogeogr. 25, 187–197 (2016).
Hallmann, K. & Griebeler, E. M. An exploration of differences in the scaling of life history traits with body mass within reptiles and between amniotes. Ecol. Evol. 8, 5480–5494 (2018).
Slavenko, A., Itescu, Y., Ihlow, F. & Meiri, S. Home is where the shell is: predicting turtle home range sizes. J. Anim. Ecol. 85, 106–114 (2016).
Regis, K. W. & Meik, J. M. Allometry of sexual size dimorphism in turtles: a comparison of mass and length data. PeerJ 5, e2914 (2017).
Itescu, Y., Karraker, N. E., Raia, P., Pritchard, P. C. H. & Meiri, S. Is the island rule general? Turtles disagree. Glob. Ecol. Biogeogr. 23, 689–700 (2014).
Faurby, S. & Svenning, J.-C. Resurrection of the island rule: human-driven extinctions have obscured a basic evolutionary pattern. Am. Nat. 187, 812–820 (2016).
Wilman, H. et al. EltonTraits 1.0: species-level foraging attributes of the world’s birds and mammals. Ecology 95, 2027–2027 (2014).
Tonini, J. F. R., Beard, K. H., Ferreira, R. B., Jetz, W. & Pyron, R. A. Fully-sampled phylogenies of squamates reveal evolutionary patterns in threat status. Biol. Conserv. 204A, 23–31 (2016).
Goolsby, E. W., Bruggeman, J. & Ané, C. Rphylopars: fast multivariate phylogenetic comparative methods for missing data and within-species variation. Methods Ecol. Evol. 8, 22–27 (2017).
Gaston, K. J., Blackburn, T. M. & Lawton, J. H. Interspecific abundance–range size relationships: an appraisal of mechanisms. J. Anim. Ecol. 66, 579–601 (1997).
Borregaard, M. K. & Rahbek, C. Causality of the relationship between geographic distribution and species abundance. Q. Rev. Biol. 85, 3–25 (2010).
IUCN Red List of Threatened Species. Version 2018 (IUCN, 2018).
Freitag, S., Hobson, C., Biggs, H. C. & Jaarsveld, A. S. Testing for potential survey bias: the effect of roads, urban areas and nature reserves on a southern African mammal data set. Anim. Conserv. 1, 119–127 (1998).
Kier, G. & Barthlott, W. Measuring and mapping endemism and species richness: a new methodological approach and its application on the flora of Africa. Biodivers. Conserv. 10, 1513–1529 (2001).
Vilela, B. & Villalobos, F. letsR: a new R package for data handling and analysis in macroecology. Methods Ecol. Evol. 6, 1229–1234 (2015).
Papavero, N. Essays on the History of Neotropical Dipterology: With Special Reference to Collectors: 1750–1905: Vol. I (Museu de Zoologia da Universidade de São Paulo, 1971).
Baselga, A., Lobo, J. M., Hortal, J., Jiménez-Valverde, A. & Gómez, J. F. Assessing alpha and beta taxonomy in eupelmid wasps: determinants of the probability of describing good species and synonyms. J. Zool. Syst. Evol. Res. 48, 40–49 (2010).
Yang, W., Ma, K. & Kreft, H. Environmental and socio-economic factors shaping the geography of floristic collections in China. Glob. Ecol. Biogeogr. 23, 1284–1292 (2014).
Karger, D. N. et al. Climatologies at high resolution for the Earth’s land surface areas. Sci. Data 4, 170122 (2017).
R Core Team R: A Language and Environment for Statistical Computing Version 3.5.3 (R Foundation for Statistical Computing, 2019).
Hijmans, R. J. raster: Geographic Data Analysis and Modeling https://cran.r-project.org/package=raster (2015).
Amatulli, G. et al. A suite of global, cross-scale topographic variables for environmental and biodiversity modeling. Sci. Data 5, 180040 (2018).
Klein Goldewijk, K., Beusen, A., Van Drecht, G. & De Vos, M. The HYDE 3.1 spatially explicit database of human-induced global land-use change over the past 12,000 years. Glob. Ecol. Biogeogr. 20, 73–86 (2011).
Joppa, L. N., Roberts, D. L. & Pimm, S. L. The population ecology and social behaviour of taxonomists. Trends Ecol. Evol. 26, 551–553 (2011).
Wickham, H. stringr: Simple, Consistent Wrappers for Common String Operations. R package version 1.3.1 http://stringr.tidyverse.org (2018).
Mahto, A. splitstackshape: Stack and Reshape Datasets After Splitting Concatenated Values. R package version 1.4.6 http://github.com/mrdwab/splitstackshape (2018).
Dinerstein, E. et al. An ecoregion-based approach to protecting half the terrestrial realm. BioScience 67, 534–545 (2017).
Kutner, M. H., Nachtsheim, C. J., Neter, J. & Li, W. Applied Linear Statistical Models (McGraw-Hill, 2004).
Naimi, B. usdm: Uncertainty Analysis for Species Distribution Models https://cran.r-project.org/package=usdm (2017).
von Linné, C. Systema Naturae https://doi.org/10.5962/bhl.title.542 (Impensis Direct Laurentii Salvii, 1758).
Harrell, F. E. Regression Modeling Strategies (Springer, 2001).
George, B., Seals, S. & Aban, I. Survival analysis and regression models. J. Nucl. Cardiol. 21, 686–694 (2014).
Jackson, C. flexsurv: a platform for parametric survival modeling in R. J. Stat. Softw. 70, 1–33 (2016).
Burnham, K. P. & Anderson, D. A. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach (Springer, 2002).
Johnson, J. B. & Omland, K. S. Model selection in ecology and evolution. Trends Ecol. Evol. 19, 101–108 (2004).
Barton, K. MuMIn: Multi-Model Inference. R package version 1.43.6 https://cran.r-project.org/package=MuMIn (2019).
Alexander Pyron, R. & Wiens, J. J. A large-scale phylogeny of Amphibia including over 2800 species, and a revised classification of extant frogs, salamanders, and caecilians. Mol. Phylogenet. Evol. 61, 543–583 (2011).
Pyron, R. A., Burbrink, F. T. & Wiens, J. J. A phylogeny and revised classification of Squamata, including 4161 species of lizards and snakes. BMC Evol. Biol. 13, 93 (2013).
Fisher, D. O. & Blomberg, S. P. Correlates of rediscovery and the detectability of extinction in mammals. Proc. R. Soc. B 278, 1090–1097 (2011).
Jetz, W., Sekercioglu, C. H. & Böhning-Gaese, K. The worldwide variation in avian clutch size across species and space. PLoS Biol. 6, e303 (2008).
Jetz, W. & Rubenstein, D. R. Environmental uncertainty and the global biogeography of cooperative breeding in birds. Curr. Biol. 21, 72–78 (2011).
Jetz, W. & Rahbek, C. Geographic range size and determinants of avian species richness. Science 297, 1548–1551 (2002).
Dowle, M. & Srinivasan, A. data.table: Extension of ‘data.frame’. R package version 1.12.4 https://cran.r-project.org/package=data.table (2019).
Gaston, K. J., Chown, S. L. & Evans, K. L. Ecogeographical rules: elements of a synthesis. J. Biogeogr. 35, 483–500 (2008).
Violle, C., Reich, P. B., Pacala, S. W., Enquist, B. J. & Kattge, J. The emergence and promise of functional biogeography. Proc. Natl Acad. Sci. USA 111, 13690–13696 (2014).
Database of Global Administrative Areas Version 3.6 (GADM, 2019); http://www.gadm.org
Acknowledgements
We are grateful to S. Meiri, D. S. Rinnan, G. Reygondeau, N. Upham, M. Costello, D. Wake and J. Hortal for providing helpful comments on the research or manuscript drafts. We thank C. Haddad, L. C. Márquez, G. Singh and A. F. Meyer for providing pictures of the example species in Fig. 1. This work was produced, in part, with the support of the National Geographic Society through a partnership with the E.O. Wilson Biodiversity Foundation and its Half-Earth Project. W. J. also acknowledges support from NSF grant DEB-1441737 and NASA grants 80NSSC17K0282 and 80NSSC18K0435.
Author information
Authors and Affiliations
Contributions
M.R.M. and W.J. conceived the study, developed the figures and wrote the text; M.R.M. analysed the data.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Ecology & Evolution thanks Joaquin Hortal, Stewart Edie and Lucas N. Joppa for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Top 30 tetrapod families with highest percentage of total future species discoveries.
a, Amphibians. b, Reptiles. c, Mammals. d, Birds. The horizontal lines denote the 95% confidence intervals. Taxon-level estimates are available through Supplementary Data 1.
Extended Data Fig. 2 Top 30 tetrapod families with highest standardized proportion of unknown species.
a, Amphibians. b, Reptiles. c, Mammals. d, Birds. The horizontal lines denote the 95% confidence intervals. Taxon-level estimates are available through Supplementary Data 1.
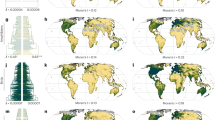
Extended Data Fig. 3 Geographical discovery patterns for terrestrial vertebrates at different spatial resolutions.
a–c, Percent of total predicted discoveries across grid cells and their respective (d–f) uncertainty (± margin of error). g–i, Standardized proportion of undiscovered species across grid cells and their respective (j–l) uncertainty (± margin of error). Outlined and hatched regions designate grid cells holding values within respectively the top 10% and top 5% of the mapped metric. Maps drawn at spatial resolutions of 220, 440, 880 km. Assemblage-level estimates are available through Supplementary Data 2.
Extended Data Fig. 4 Geographical discovery patterns for amphibians at different spatial resolutions.
a–c, Percent of total discoveries across grid cells and their respective (d–f) uncertainty (± margin of error). g–i, Standardized proportion of undiscovered species across grid cells and their respective (j–l) uncertainty (± margin of error). Outlined and hatched regions designate grid cells holding values within respectively the top 10% and top 5% of the mapped metric. Maps drawn at spatial resolutions of 220, 440, 880 km. Assemblage-level estimates are available through Supplementary Data 2.
Extended Data Fig. 5 Geographical discovery patterns for reptiles at different spatial resolutions.
a–c, Percent of total discoveries across grid cells and their respective (d-f) uncertainty (± margin of error). (g–i) Standardized proportion of undiscovered species across grid cells and their respective (j–l) uncertainty (± margin of error). Outlined and hatched regions designate grid cells holding values within respectively the top 10% and top 5% of the mapped metric. Maps drawn at spatial resolutions of 220, 440, 880 km. Assemblage-level estimates are available through Supplementary Data 2.
Extended Data Fig. 6 Geographical discovery patterns for mammals at different spatial resolutions.
a–c, Percent of total discoveries across grid cells and their respective (d-f) uncertainty (± margin of error). g–i, Standardized proportion of undiscovered species across grid cells and their respective (j–l) uncertainty (± margin of error). Outlined and hatched regions designate grid cells holding values within respectively the top 10% and top 5% of the mapped metric. Maps drawn at spatial resolutions of 220, 440, 880 km. Assemblage-level estimates are available through Supplementary Data 2.
Extended Data Fig. 7 Geographical discovery patterns for birds at different spatial resolutions.
a–c, Percent of total discoveries across grid cells and their respective (d-f) uncertainty (± margin of error). g–i, Standardized proportion of undiscovered species across grid cells and their respective (j–l) uncertainty (± margin of error). Outlined and hatched regions designate grid cells holding values within respectively the top 10% and top 5% of the mapped metric. Maps drawn at spatial resolutions of 220, 440, 880 km. Assemblage-level estimates are available through Supplementary Data 2.
Extended Data Fig. 8 Biogeographical realms and biomes with higher percent of total future discoveries.
Biogeographic- and Biome-wide percent of total discoveries extracted from assemblages defined at (a) 220 km, (b) 440 km, and (c) 880 km of spatial resolution. Bioregion-level estimates are available through Supplementary Data 3.
Extended Data Fig. 9 Top 30 bioregions with higher percent of total future discoveries.
Bioregions-wide percent of total discoveries extracted from assemblages defined at (a) 220 km, (b) 440 km, and (c) 880 km of spatial resolution. A bioregion combines biogeographical realm and biome information. Bioregion-level estimates are available through Supplementary Data 3.
Extended Data Fig. 10 Top 30 countries with higher percent of total discoveries.
Country-wide percent of total discoveries extracted from assemblages defined at (a) 220 km, (b) 440 km, and (c) 880 km of spatial resolution. Country-level estimates are available through Supplementary Data 4.
Supplementary information
Supplementary Information
Supplementary Methods, Results, References, Tables 1–4 and Figs. 1–15.
Supplementary Data
Estimates of discovery potential at the levels of taxa, assemblages, bioregions and countries: TaxonLevelEstimates.zip (Supplementary Data 1), AssemblageLevelEstimates.zip (Supplementary Data 2), BioregionLevelEstimates.zip (Supplementary Data 3), CountryLevelEstimates.zip (Supplementary Data 4).
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Moura, M.R., Jetz, W. Shortfalls and opportunities in terrestrial vertebrate species discovery. Nat Ecol Evol 5, 631–639 (2021). https://doi.org/10.1038/s41559-021-01411-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41559-021-01411-5
This article is cited by
-
Reply to: Estimates of the number of undescribed species should account for sampling effort
Nature Ecology & Evolution (2024)
-
Estimates of the number of undescribed species should account for sampling effort
Nature Ecology & Evolution (2024)
-
Caught in the crossfire: biodiversity conservation paradox of sociopolitical conflict
npj Biodiversity (2024)
-
Evidence on the efficacy of small unoccupied aircraft systems (UAS) as a survey tool for North American terrestrial, vertebrate animals: a systematic map
Environmental Evidence (2023)
-
Global shortfalls of knowledge on anuran tadpoles
npj Biodiversity (2023)