Abstract
The metabotropic gamma-aminobutyric acid B receptor (GABABR) is a G protein-coupled receptor that mediates neuronal inhibition by the neurotransmitter GABA. While GABABR-mediated signalling has been suggested to play central roles in neuronal differentiation and proliferation across evolution, it has mostly been studied in the mammalian brain. Here, we demonstrate that ectopic activation of GABABR signalling affects neurogenic functions in the sea anemone Nematostella vectensis. We identified four putative Nematostella GABABR homologues presenting conserved three-dimensional extracellular domains and residues needed for binding GABA and the GABABR agonist baclofen. Moreover, sustained activation of GABABR signalling reversibly arrests the critical metamorphosis transition from planktonic larva to sessile polyp life stage. To understand the processes that underlie the developmental arrest, we combined transcriptomic and spatial analyses of control and baclofen-treated larvae. Our findings reveal that the cnidarian neurogenic programme is arrested following the addition of baclofen to developing larvae. Specifically, neuron development and neurite extension were inhibited, resulting in an underdeveloped and less organized nervous system and downregulation of proneural factors including NvSoxB(2), NvNeuroD1 and NvElav1. Our results thus point to an evolutionarily conserved function of GABABR in neurogenesis regulation and shed light on early cnidarian development.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Transcriptome datasets used in this study are available via the SRA database with accession no. SRP140400.
References
Ulrich, D. & Bettler, B. GABAB receptors: synaptic functions and mechanisms of diversity. Curr. Opin. Neurobiol. 17, 298–303 (2007).
Kaupmann, K. et al. GABA B-receptor subtypes assemble into functional heteromeric complexes. Nature 396, 683–687 (1998).
Jones, K. A. et al. GABAB receptors function as a heteromeric assembly of the subunits GABABR1 and GABABR2. Nature 396, 674–679 (1998).
White, J. H. et al. Heterodimerization is required for the formation of a functional GABA B receptor. Nature 396, 679–682 (1998).
Geng, Y., Bush, M., Mosyak, L., Wang, F. & Fan, Q. R. Structural mechanism of ligand activation in human GABAB receptor. Nature 504, 254–259 (2013).
Galvez, T. et al. Mutagenesis and modeling of the GABAB receptor extracellular domain support a venus flytrap mechanism for ligand binding. J. Biol. Chem. 274, 13362–13369 (1999).
Margeta-Mitrovic, M., Jan, Y. N. & Jan, L. Y. A trafficking checkpoint controls GABAB receptor heterodimerization. Neuron 27, 97–106 (2000).
Robbins, M. J. et al. GABAB2 is essential for G-protein coupling of the GABAB receptor heterodimer. J. Neurosci. 21, 8043–8052 (2001).
Kniazeff, J., Galvez, T., Labesse, G. & Pin, J. P. No ligand binding in the GB2 subunit of the GABA(B) receptor is required for activation and allosteric interaction between the subunits. J. Neurosci. 22, 7352–7361 (2002).
Liu, J. et al. Molecular determinants involved in the allosteric control of agonist affinity in the GABAB receptor by the GABAB2 subunit. J. Biol. Chem. 279, 15824–15830 (2004).
Felder, C. B., Graul, R. C., Lee, A. Y., Merkle, H. P. & Sadee, W. The Venus flytrap of periplasmic binding proteins: an ancient protein module present in multiple drug receptors. AAPS PharmSciTech. 1, 7–26 (1999).
Kilb, W., Kirischuk, S. & Luhmann, H. Role of tonic GABAergic currents during pre- and early postnatal rodent development. Front. Neural Circuits 7, 139 (2013).
Fukui, M. et al. Modulation of cellular proliferation and differentiation through GABAB receptors expressed by undifferentiated neural progenitor cells isolated from fetal mouse brain. J. Cell. Physiol. 216, 507–519 (2008).
Gaiarsa, J.-L., Kuczewski, N. & Porcher, C. Contribution of metabotropic GABAB receptors to neuronal network construction. Pharmacol. Ther. 132, 170–179 (2011).
Bony, G. et al. Non-hyperpolarizing GABAB receptor activation regulates neuronal migration and neurite growth and specification by cAMP/LKB1. Nat. Commun. 4, 1800 (2013).
Giachino, C. et al. GABA suppresses neurogenesis in the adult hippocampus through GABAB receptors. Development 141, 83–90 (2014).
Sibbe, M. & Kulik, A. GABAergic regulation of adult hippocampal neurogenesis. Mol. Neurobiol. 54, 5497–5510 (2017).
Dittman, J. S. & Kaplan, J. M. Behavioral impact of neurotransmitter-activated G-protein-coupled receptors: muscarinic and GABAB receptors regulate Caenorhabditis elegans locomotion. J. Neurosci. 28, 7104–7112 (2008).
Mezler, M., Müller, T. & Raming, K. Cloning and functional expression of GABAB receptors from Drosophila. Eur. J. Neurosci. 13, 477–486 (2001).
Colombo, G. (ed.) GABAB Receptor Vol. 29 (Springer, 2016).
Blankenburg, S. et al. Cockroach GABAB receptor subtypes: molecular characterization, pharmacological properties and tissue distribution. Neuropharmacology 88, 134–144 (2015).
Rentzsch, F., Layden, M. & Manuel, M. The cellular and molecular basis of cnidarian neurogenesis. Wiley Interdiscip. Rev. Dev. Biol. 6, e257 (2016).
Ryan, J. F. & Chiodin, M. Where is my mind? How sponges and placozoans may have lost neural cell types. Phil. Trans. R. Soc. B 370, 20150059 (2015).
Nickel, M. Evolutionary emergence of synaptic nervous systems: what can we learn from the non-synaptic, nerveless Porifera? Invertebr. Biol. 129, 1–16 (2010).
Watanabe, H. in Brain Evolution by Design: From Neural Origin to Cognitive Architecture (eds Shigeno, S. et al.) 45–75 (Springer Japan, 2017).
Moroz, L. L. & Kohn, A. B. Independent origins of neurons and synapses: insights from ctenophores. Phil. Trans. R. Soc. Lond. B. 371, 20150041 (2016).
Kelava, I., Rentzsch, F. & Technau, U. Evolution of eumetazoan nervous systems: insights from cnidarians. Phil. Trans. R. Soc. Lond. B. 370, 20150065 (2015).
Galliot, B. & Quiquand, M. A two-step process in the emergence of neurogenesis. Eur. J. Neurosci. 34, 847–862 (2011).
Layden, M. J., Rentzsch, F. & Röttinger, E. The rise of the starlet sea anemone Nematostella vectensis as a model system to investigate development and regeneration. Wiley Interdiscip. Rev. Dev. Biol. 5, 408–428 (2016).
Rentzsch, F., Juliano, C. & Galliot, B. Modern genomic tools reveal the structural and cellular diversity of cnidarian nervous systems. Curr. Opin. Neurobiol. 56, 87–96 (2019).
Rentzsch, F. & Technau, U. Genomics and development of Nematostella vectensis and other anthozoans. Curr. Opin. Genet. Dev. 39, 63–70 (2016).
Putnam, N. et al. Sea anemone genome reveals ancestral eumetazoan gene repertoire and genomic organization. Science 317, 86–94 (2007).
Darling, J. A. et al. Rising starlet: the starlet sea anemone, Nematostella vectensis. Bioessays 27, 211–221 (2005).
Anctil, M. Chemical transmission in the sea anemone Nematostella vectensis: a genomic perspective. Comp. Biochem. Physiol. D 4, 268–289 (2009).
Bosch, T. C. G. et al. Back to the basics: cnidarians start to fire. Trends Neurosci. 40, 92–105 (2017).
Marlow, H. Q., Srivastava, M., Matus, D. Q., Rokhsar, D. & Martindale, M. Q. Anatomy and development of the nervous system of Nematostella vectensis, an anthozoan cnidarian. Dev. Neurobiol. 69, 235–254 (2009).
Faltine-Gonzalez, D. Z. & Layden, M. J. Characterization of nAChRs in Nematostella vectensis supports neuronal and non-neuronal roles in the cnidarian–bilaterian common ancestor. EvoDevo 10, 27 (2019).
Adams, C. & Lawrence, A. CGP7930: a positive allosteric modulator of the GABAB receptor. CNS Drug Rev. 13, 308–316 (2007).
Lee, P. N., Pang, K., Matus, D. Q. & Martindale, M. Q. A. WNT of things to come: evolution of Wnt signaling and polarity in cnidarians. Semin. Cell Dev. Biol. 17, 157–167 (2006).
Trevino, M., Stefanik, D. J., Rodriguez, R., Harmon, S. & Burton, P. M. Induction of canonical Wnt signaling by alsterpaullone is sufficient for oral tissue fate during regeneration and embryogenesis in Nematostella vectensis. Dev. Dyn. 240, 2673–2679 (2011).
Kusserow, A. et al. Unexpected complexity of the Wnt gene family in a sea anemone. Nature 433, 156–160 (2005).
Gurevich, V. V. & Gurevich, E. V. How and why do GPCRs dimerize? Trends Pharmacol. Sci. 29, 234–240 (2008).
Calver, A. R. et al. The C-terminal domains of the GABAB receptor subunits mediate intracellular trafficking but are not required for receptor signaling. J. Neurosci. 21, 1203–1210 (2001).
Grünewald, S. et al. Importance of the γ-aminobutyric acidB receptor C-termini for G-protein coupling. Mol. Pharmacol. 61, 1070–1080 (2002).
Pagano, A. et al. C-terminal interaction is essential for surface trafficking but not for heteromeric assembly of GABAB receptors. J. Neurosci. 21, 1189–1202 (2001).
Sebé-Pedrós, A. et al. Cnidarian cell type diversity revealed by whole-organism single-cell RNA-seq analysis. Cell 173, 1520–1534 (2018).
Karabulut, A., He, S., Chen, C.-Y., McKinney, S. A. & Gibson, M. C. Electroporation of short hairpin RNAs for rapid and efficient gene knockdown in the starlet sea anemone, Nematostella vectensis. Dev. Biol. 448, 7–15 (2019).
He, S. et al. An axial Hox code controls tissue segmentation and body patterning in Nematostella vectensis. Science 361, 1377–1380 (2018).
Benke, D. in Advances in Pharmacology (ed. Blackburn, T. P.) 93–111 (Elsevier, 2010).
Kantamneni, S. et al. GISP binding to TSG101 increases GABAB receptor stability by down‐regulating ESCRT‐mediated lysosomal degradation. J. Neurochem. 107, 86–95 (2008).
Kantamneni, S., Holman, D., Wilkinson, K. A., Nishimune, A. & Henley, J. M. GISP increases neurotransmitter receptor stability by down-regulating ESCRT-mediated lysosomal degradation. Neurosci. Lett. 452, 106–110 (2009).
Magie, C., Pang, K. & Martindale, M. Genomic inventory and expression of Sox and Fox genes in the cnidarian Nematostella vectensis. Dev. Genes Evol. 215, 618–630 (2005).
Richards, G. S. & Rentzsch, F. Transgenic analysis of a SoxB gene reveals neural progenitor cells in the cnidarian Nematostella vectensis. Development 141, 4681–4689 (2014).
Layden, M. J., Boekhout, M. & Martindale, M. Q. Nematostella vectensis achaete-scute homolog NvashA regulates embryonic ectodermal neurogenesis and represents an ancient component of the metazoan neural specification pathway. Development 139, 1013–1022 (2012).
Richards, G. S. & Rentzsch, F. Regulation of Nematostella neural progenitors by SoxB, Notch and bHLH genes. Development 142, 3332–3342 (2015).
Layden, M. J. et al. MAPK signaling is necessary for neurogenesis in Nematostella vectensis. BMC Biol. 14, 61 (2016).
Watanabe, H. et al. Sequential actions of β-catenin and Bmp pattern the oral nerve net in Nematostella vectensis. Nat. Commun. 5, 5536 (2014).
Mazza, M. E., Pang, K., Martindale, M. Q. & Finnerty, J. R. Genomic organization, gene structure, and developmental expression of three clustered otx genes in the sea anemone Nematostella vectensis. J. Exp. Zool. B 308, 494–506 (2007).
Nakanishi, N., Renfer, E., Technau, U. & Rentzsch, F. Nervous systems of the sea anemone Nematostella vectensis are generated by ectoderm and endoderm and shaped by distinct mechanisms. Development 139, 347–357 (2012).
Marlow, H., Roettinger, E., Boekhout, M. & Martindale, M. Q. Functional roles of Notch signaling in the cnidarian Nematostella vectensis. Dev. Biol. 362, 295–308 (2012).
Babonis, L. S. & Martindale, M. Q. PaxA, but not PaxC, is required for cnidocyte development in the sea anemone Nematostella vectensis. EvoDevo 8, 14 (2017).
Zenkert, C., Takahashi, T., Diesner, M.-O. & Özbek, S. Morphological and molecular analysis of the Nematostella vectensis cnidom. PLoS ONE 6, e22725 (2011).
Sunagar, K. et al. Cell type-specific expression profiling unravels the development and evolution of stinging cells in sea anemone. BMC Biol. 16, 108 (2018).
Matus, D. Q., Pang, K., Daly, M. & Martindale, M. Q. Expression of Pax gene family members in the anthozoan cnidarian, Nematostella vectensis. Evol. Dev. 9, 25–38 (2007).
Zatylny-Gaudin, C. & Favrel, P. Diversity of the RFamide peptide family in mollusks. Front. Endocrinol. (Lausanne) 5, 178 (2014).
Bause, M., van der Horst, R. & Rentzsch, F. Glypican1/2/4/6 and sulfated glycosaminoglycans regulate the patterning of the primary body axis in the cnidarian Nematostella vectensis. Dev. Biol. 414, 108–120 (2016).
Rentzsch, F., Fritzenwanker, J. H., Scholz, C. B. & Technau, U. FGF signalling controls formation of the apical sensory organ in the cnidarian Nematostella vectensis. Development 135, 1761–1769 (2008).
Hozumi, A. et al. GABA-Induced GnRH release triggers chordate metamorphosis. Curr. Biol. 30, 1555–1561 (2020).
Biscocho, D., Cook, J. G., Long, J., Shah, N. & Leise, E. M. GABA is an inhibitory neurotransmitter in the neural circuit regulating metamorphosis in a marine snail. Dev. Neurobiol. 78, 736–753 (2018).
Joyce, A. & Vogeler, S. Molluscan bivalve settlement and metamorphosis: neuroendocrine inducers and morphogenetic responses. Aquaculture 487, 64–82 (2018).
Rahmani, M. & Uehara, T. Induction of metamorphosis and substratum preference in four sympatric and closely related species of sea urchins (Genus Echinometra) in Okinawa. Zool. Stud. 40, 29–43 (2001).
Scappaticci, A. A. & Kass-Simon, G. NMDA and GABAB receptors are involved in controlling nematocyst discharge in hydra. Comp. Biochem. Physiol. A 150, 415–422 (2008).
Lauro, B. M. & Kass-Simon, G. Hydra’s feeding response: effect of GABAB ligands on GSH-induced electrical activity in the hypostome of H. vulgaris. Comp. Biochem. Physiol. A 225, 83–93 (2018).
Schaeffer, J. M. & Hsueh, A. J. Identification of gamma-aminobutyric acid and its binding sites in the rat ovary. Life Sci. 30, 1599–1604 (1982).
Shen, W., Nan, C., Nelson, P. T., Ripps, H. & Slaughter, M. M. GABAB receptor attenuation of GABAA currents in neurons of the mammalian central nervous system. Physiol. Rep. 5, e13129 (2017).
Shim, J. et al. Olfactory control of blood progenitor maintenance. Cell 155, 1141–1153 (2013).
Sarkar, A. & Hochedlinger, K. The Sox family of transcription factors: versatile regulators of stem and progenitor cell fate. Cell Stem Cell 12, 15–30 (2013).
Bylund, M., Andersson, E., Novitch, B. G. & Muhr, J. Vertebrate neurogenesis is counteracted by Sox1–3 activity. Nat. Neurosci. 6, 1162–1168 (2003).
Pevny, L. & Placzek, M. SOX genes and neural progenitor identity. Curr. Opin. Neurobiol. 15, 7–13 (2005).
Wegner, M. SOX after SOX: SOXession regulates neurogenesis. Genes Dev. 25, 2423–2428 (2011).
Whittington, N., Cunningham, D., Le, T.-K., De Maria, D. & Silva, E. M. Sox21 regulates the progression of neuronal differentiation in a dose-dependent manner. Dev. Biol. 397, 237–247 (2015).
Royo, J. L. et al. Transphyletic conservation of developmental regulatory state in animal evolution. Proc. Natl Acad. Sci. USA 108, 14186–14191 (2011).
Jager, M., Quéinnec, E., Le Guyader, H. & Manuel, M. Multiple Sox genes are expressed in stem cells or in differentiating neuro-sensory cells in the hydrozoan Clytia hemisphaerica. EvoDevo 2, 12 (2011).
Schnitzler, C. E., Simmons, D. K., Pang, K., Martindale, M. Q. & Baxevanis, A. D. Expression of multiple Sox genes through embryonic development in the ctenophore Mnemiopsis leidyi is spatially restricted to zones of cell proliferation. EvoDevo 5, 15 (2014).
Steinmetz, P. R. H., Aman, A., Kraus, J. E. M. & Technau, U. Gut-like ectodermal tissue in a sea anemone challenges germ layer homology. Nat. Ecol. Evol. 1, 1535–1542 (2017).
Busengdal, H. & Rentzsch, F. Unipotent progenitors contribute to the generation of sensory cell types in the nervous system of the cnidarian Nematostella vectensis. Dev. Biol. 431, 59–68 (2017).
Elran, R. et al. Early and late response of Nematostella vectensis transcriptome to heavy metals. Mol. Ecol. 23, 4722–4736 (2014).
Fritzenwanker, J. & Technau, U. Induction of gametogenesis in the basal cnidarian Nematostella vectensis (Anthozoa). Dev. Genes Evol. 212, 99–103 (2002).
Bordoli, L. et al. Protein structure homology modeling using SWISS-MODEL workspace. Nat. Protoc. 4, 1–13 (2008).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Love, M.I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15, 550 (2014).
Khomtchouk, B. B., Hennessy, J. R. & Wahlestedt, C. shinyheatmap: ultra fast low memory heatmap web interface for big data genomics. PLoS ONE 12, e0176334 (2017).
Young, M. D., Wakefield, M. J., Smyth, G. K. & Oshlack, A. Gene ontology analysis for RNA-seq: accounting for selection bias. Genome Biol. 11, R14 (2010).
Wolenski, F. S., Layden, M. J., Martindale, M. Q., Gilmore, T. D. & Finnerty, J. R. Characterizing the spatiotemporal expression of RNAs and proteins in the starlet sea anemone, Nematostella vectensis. Nat. Protoc. 8, 900–915 (2013).
Genikhovich, G. & Technau, U. Anti-acetylated tubulin antibody staining and phalloidin staining in the starlet sea anemone Nematostella vectensis. Cold Spring Harb. Protoc. 2009, pdb.prot5283 (2009).
Gassmann, M. & Bettler, B. Regulation of neuronal GABAB receptor functions by subunit composition. Nat. Rev. Neurosci. 13, 380–394 (2012).
Acknowledgements
We thank F. Rentzsch for providing the NvElav1 reporter line. We thank the Bioinformatics Service Unit at the University of Haifa and, specifically, N. Sher and M. Lalzar for their assistance. We thank S. Ben-Tabou de-Leon for helpful comments. This work was supported by the Israel Ministry of Science and Technology (grant no. 3-8774), the Israel Science Foundation (grant nos. 1454/13, 2155/15 and 3512/19) and the DS Research Centre at the University of Haifa.
Author information
Authors and Affiliations
Contributions
S.L. designed and performed experiments. V.B. performed gene cloning, shRNA knockdown and assisted in experiments. S.L., A.B. and M.K. performed sequence and structure analysis. A.M. performed bioinformatics analyses. A.S.-P. analysed GABABR homologue and TF expression in the single-cell dataset. S.L., V.B., M.K. and T.L. analysed the data. M.K. supervised sequence and structure analysis. T.L. conceived and supervised the project and wrote the manuscript with M.K. and input from all authors. All authors discussed the results and commented on the manuscript. All authors read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
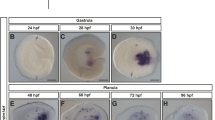
Extended Data Fig. 1 GABABR modulators inhibit planulae-to-polyp transformation.
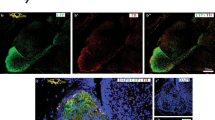
Confocal sections at 5 dpf (a-d) and 8 dpf (i-l) labeled with antibodies against phalloidin (green) and DAPI (blue). DIC images of the aboral/apical tuft at 5 dpf (e–h) and 8 dpf (m-p). Control planulae (a,i) and primary polyps (i, m) are shown, as are planulae treated with GABA (b, f, j, n), baclofen (c, g, k, o) or CGP-7930 (d, h, I, p). While at 5 dpf all planulae possessed an apical tuft (numbers are not shown), in 8 dpf control primary polyps after metamorphosis (m), the apical tuft was lost, whereas treated 8 dpf planulae (n–p) still maintained it. The fraction of similar phenotypes from the total number of analyzed samples is given in the lower right-hand corner. Scale bars, 50 µm.
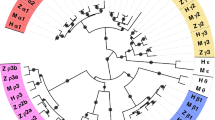
Extended Data Fig. 2 Schematic representation of predicted domains in putative Nematostella GABABR homologs in comparison to human GABAB1R.
The eight Nematostella proteins contain a conserved signal peptide, an extracellular ‘Periplasmic Binding Protein type1 (PBP1) GABAB ligand-binding domain’ (the structural VFT module that in mammals binds GABA), predicted helical transmembrane domains, and coiled-coil domains. One Nematostella homolog (v1g206093) contained two extracellular domains, each corresponding to a separate predicted VFT module. These two domains are 26% identical in sequence, suggesting that they serve dissimilar functions (Supplementary Fig. 2). v1g243252 contains eight predicted TM helices and a ~300 residue domain of unknown function (DUF4475) located after these TM domains. However, the intracellular C-terminal domains of the Nematostella homologs present low similarity to the corresponding regions of human sequences. Coiled-coil motifs found in the C-terminus of human GABABR were predicted in three Nematostella homologs. The mammalian GABABR C-terminal domain mediate processes such as trafficking out of the ER or modulation of receptor activity44, but it has also been suggested as non-essential for functional GABABR heterodimers42,43,45. GABABR C-termini therefore differ dramatically between mammals and cnidarians, suggesting they do not affect essential functions, and were excluded from the full comparison. Protein domains were predicted as detailed in Methods.
Supplementary information
Supplementary Information
Supplementary Figs. 1–5, Methods and Table 3.
Supplementary Table 1
Transcriptome analysis of differentially expressed transcripts of control and baclofen-treated planulae.
Supplementary Table 2
GOSeq analysis of differentially expressed transcripts of control and baclofen-treated planulae.
Rights and permissions
About this article
Cite this article
Levy, S., Brekhman, V., Bakhman, A. et al. Ectopic activation of GABAB receptors inhibits neurogenesis and metamorphosis in the cnidarian Nematostella vectensis. Nat Ecol Evol 5, 111–121 (2021). https://doi.org/10.1038/s41559-020-01338-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41559-020-01338-3