Abstract
Nonlinear dynamics, where a change in the input is not proportional to a change in the output, are often found throughout nature, for example in biochemical kinetics. Because of the complex suite of interacting abiotic and biotic variables present in ecosystems, animal population dynamics are often thought to be driven in a nonlinear, state-dependent fashion. However, so far these have only been identified in model organisms and some natural systems. Here we show that nonlinear population dynamics are ubiquitous in nature. We use nonlinear forecasting to analyse 747 datasets of 228 species to find that insect population trends were highly nonlinear (74%), followed by mammals (58%), bony fish (49%) and birds (35%). This indicates that linear, equilibrium-based model assumptions may fail at predicting population dynamics across a wide range of animal taxa. We show that faster-reproducing animals are more likely to have nonlinear and high-dimensional dynamics, supporting past ecological theory. Lastly, only a third of time series were predictable beyond two years; therefore, the ability to predict animal population trends using these methods may be limited. Our results suggest that the complex dynamics necessary to cause regime shifts and other transitions may be inherent in a wide variety of animals.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The data needed to reproduce the analysis can be found on Github (https://doi.org/10.5281/zenodo.3470260).
Code availability
The code needed to reproduce the analysis can be found on Github (https://doi.org/10.5281/zenodo.3470260).
References
Scott, A. C. The Nonlinear Universe: Chaos, Emergence, Life (Springer, 2007).
Schaffer, W. M. & Kot, M. Do strange attractors govern ecological systems? BioScience 35, 342–350 (1985).
Pascual, M. & Ellner, S. P. Linking ecological patterns to environmental forcing via nonlinear time series. Ecology 81, 2767–2780 (2000).
Hsieh, C. H., Glaser, S. M., Lucas, A. J. & Sugihara, G. Distinguishing random environmental fluctuations from ecological catastrophes for the North Pacific Ocean. Nature 435, 336–340 (2005).
May, R. M. & Oster, G. F. Bifurcations and dynamic complexity in simple ecological models. Am. Nat. 110, 573–599 (1976).
Glaser, S. M. et al. Complex dynamics may limit prediction in marine fisheries. Fish Fish. (Oxf) 15, 616–633 (2014).
May, R. M. Simple mathematical models with very complicated dynamics. Nature 261, 459–467 (1976).
Turchin, P. & Taylor, A. D. Complex dynamics in ecological time series. Ecology 73, 289–305 (1992).
Hassell, M. P., Lawton, J. H. & May, R. M. Patterns of dynamical behaviour in single-species populations. J. Anim. Ecol. 45, 471–486 (1976).
Mueller, L. D. & Joshi, A. Stability in Model Populations (Princeton Univ. Press, 2000).
Dennis, B., Desharnais, R. A., Cushing, J. M., Henson, S. M. & Costantino, R. F. Estimating chaos and complex dynamics in an insect population. Ecol. Monogr. 71, 277–304 (2001).
May, R. M. Biological populations with nonoverlapping generations: stable points, stable cycles, and chaos. Science 186, 645–647 (1974).
Dixon, P. A., Milicich, M. & Sugihara, G. Episodic fluctuations in larval supply. Science 283, 1528–1530 (1999).
Sugihara, G. Nonlinear forecasting for the classification of natural time series. Philos. Trans. Royal Soc. A 348, 477–495 (1994).
May, R. M., Beddington, J. R., Clark, C. W., Holt, S. J. & Laws, R. M. Management of multispecies fisheries. Science 205, 267–277 (1979).
Fogarty, M. J., Gamble, R. & Perretti, C. T. Dynamic complexity in exploited marine ecosystems. Front. Ecol. Evol. 4, 68 (2016).
Klein, E. S., Glaser, S. M., Jordaan, A., Kaufman, L. & Rosenberg, A. A. A complex past: historical and contemporary fisheries demonstrate nonlinear dynamics and a loss of determinism. Mar. Ecol. Prog. Ser. 557, 237–246 (2016).
Polis, G. A., Sears, A. L. W., Huxel, G. R., Strong, D. R. & Maron, J. When is a trophic cascade a trophic cascade? Trends Ecol. Evol. 15, 473–475 (2000).
Shurin, J. B. et al. A cross-ecosystem comparison of the strength of trophic cascades. Ecol. Lett. 5, 785–791 (2002).
McCann, K., Hastings, A. & Huxel, G. Weak trophic interactions and the balance of nature. Nature 395, 794–798 (1998).
Ziebarth, N. L., Abbott, K. C. & Ives, A. R. Weak population regulation in ecological time series. Ecol. Lett. 13, 21–31 (2010).
Zhou, X. et al. Detecting chaotic dynamics of insect populations from long-term survey data. Ecol. Entomol. 22, 231–241 (1997).
Leibold, M. A. & Chase, J. M. Metacommunity Ecology (Princeton Univ. Press, 2018).
de Ruiter, P. C., Neutel, A. M. & Moore, J. C. Energetics, patterns of interaction strengths, and stability in real ecosystems. Science 269, 1257–1260 (1995).
Scheffer, M., Carpenter, S., Foley, J. A., Folke, C. & Walker, B. Catastrophic shifts in ecosystems. Nature 413, 591–596 (2001).
Scheffer, M. et al. Early-warning signals for critical transitions. Nature 461, 53–59 (2009).
Dakos, V., Glaser, S. M., Hsieh, C. & Sugihara, G. Elevated nonlinearity as an indicator of shifts in the dynamics of populations under stress. J. R. Soc. Interface 14, 20160845 (2017).
Bjørnstad, O. N. & Grenfell, B. T. Noisy clockwork: time series analysis of population fluctuations in animals. Science 293, 638–643 (2001).
Coulson, T., Rohani, P. & Pascual, M. Skeletons, noise and population growth: the end of an old debate? Trends Ecol. Evol. 19, 359–364 (2004).
Hsieh, C. H., Anderson, C. & Sugihara, G. Extending nonlinear analysis to short ecological time series. Am. Nat. 171, 71–80 (2008).
Sugihara, G. & May, R. M. Nonlinear forecasting as a way of distinguishing chaos from measurement error in time series. Nature 344, 734–741 (1990).
Ye, H. et al. Equation-free mechanistic ecosystem forecasting using empirical dynamic modeling. Proc. Natl Acad. Sci. USA 112, E1569–E1576 (2015).
Giron-Nava, A. et al. Quantitative argument for long-term ecological monitoring. Mar. Ecol. Prog. Ser. 572, 269–274 (2017).
Ebisuzaki, W. A method to estimate the statistical significance of a correlation when the data are serially correlated.J. Clim. 10, 2147–2153 (1997).
Herrando-Pérez, S., Delean, S., Brook, B. W. & Bradshaw, C. J. A. Strength of density feedback in census data increases from slow to fast life histories. Ecol. Evol. 2, 1922–1934 (2012).
Brook, B. W., Traill, L. W. & Bradshaw, C. J. Minimum viable population sizes and global extinction risk are unrelated. Ecol. Lett. 9, 375–382 (2006).
Costantino, R. F., Desharnais, R. A., Cushing, J. & Dennis, B. Chaotic dynamics in an insect population. Science 275, 389–391 (1997).
Turchin, P. & Ellner, S. P. Living on the edge of chaos: population dynamics of Fennoscandian voles. Ecology 81, 3099–3116 (2000).
Takens, F. Detecting strange attractors in turbulence. In Proc. Symposium on Dynamical Systems and Turbulence (eds Rand, D. & Young, L.) 366–381 (Springer, 1981).
Ward, E. J., Holmes, E. E., Thorson, J. T. & Collen, B. Complexity is costly: a meta-analysis of parametric and non-parametric methods for short-term population forecasting. Oikos 123, 652–661 (2014).
Mouquet, N. et al. Predictive ecology in a changing world. J. Appl. Ecol. 52, 1293–1310 (2015).
Petchey, O. L. et al. The ecological forecast horizon, and examples of its uses and determinants. Ecol. Lett. 18, 597–611 (2015).
Knape, J. & de Valpine, P. Are patterns of density dependence in the Global Population Dynamics Database driven by uncertainty about population abundance? Ecol. Lett. 15, 17–23 (2012).
Perretti, C. T., Sugihara, G. & Munch, S. B. Nonparametric forecasting outperforms parametric methods for a simulated multispecies system. Ecology 94, 794–800 (2013).
Cenci, S., Sugihara, G. & Saavedra, S. Regularized S-map for inference and forecasting with noisy ecological time series. Methods Ecol. Evol. 10, 650–660 (2019).
Ahrestani, F. S., Hebblewhite, M. & Post, E. The importance of observation versus process error in analyses of global ungulate populations. Sci. Rep. 3, 3125 (2013).
Graham, N. A., Jennings, S., MacNeil, M. A., Mouillot, D. & Wilson, S. K. Predicting climate-driven regime shifts versus rebound potential in coral reefs. Nature 518, 94–97 (2015).
Drake, J. M. & Griffen, B. D. Early warning signals of extinction in deteriorating environments. Nature 467, 456–459 (2010).
Fryxell, J. M., Packer, C., McCann, K., Solberg, E. J. & Saether, B. E. Resource management cycles and the sustainability of harvested wildlife populations. Science 328, 903–906 (2010).
Keith, D. et al. Temporal correlations in population trends: conservation implications from time-series analysis of diverse animal taxa. Biol. Conserv. 192, 247–257 (2015).
Hinchliff, C. E. et al. Synthesis of phylogeny and taxonomy into a comprehensive tree of life. Proc. Natl Acad. Sci. USA 112, 12764–12769 (2015).
Michonneau, F., Brown, J. W. & Winter, D. J. rotl: an R package to interact with the Open Tree of Life data. Methods Ecol. Evol. 7, 1476–1481 (2016).
R Core Team. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2018).
Ye, H. et al. rEDM: Applications of empirical dynamic modeling from time series. R package version 0.7.5 https://github.com/ha0ye/rEDM (2018).
Paradis, E., Claude, J. & Strimmer, K. APE: Analyses of Phylogenetics and Evolution in R language. Bioinformatics. 20, 289–290 (2004).
Gelman, A., Jakulin, A., Pittau, M. G. & Su, Y.-S. A weakly informative default prior distribution for logistic and other regression models. Ann. Appl. Stat. 2, 1360–1383 (2008).
Gelman, A. et al. arm: Data analysis using regression and multilevel/hiearchical models. R package version 1.10-1 https://CRAN.R-project.org/package=arm (2018).
Acknowledgements
Funding for this project was provided by the National Science Foundation (NSF) Graduate Research Fellowship under grant no. 366280 (T.J.C.), the Idaho Department of Fish and Game (T.J.C.) and NSF grant no. 1836793 (A.D.L.). We thank H. Wilhelm Martin for his superb assistance with the analysis and M. Hebblewhite and J. Maron for comments on earlier drafts.
Author information
Authors and Affiliations
Contributions
T.J.C. collected and analysed the data and wrote the manuscript. A.D.L. supervised the project and edited the manuscript.
Corresponding author
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Principal components analysis of life-history traits.
PC1 explains 72.2% of variation in our data (axes 1, 2, and 3), representing body length (mm), minimum age at first reproduction (months), and lifespan (months), respectively. PC2 explains 24.9% of variation in our data (axis 4), representing fertility (# of young per year). Colored ellipses represent 95% probability that data for each taxonomic classification fall within the ellipse.
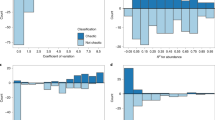
Extended Data Fig. 2 Final model results of nonlinearity.
E is the embedding dimensionality, ρ is the forecast skill, and CV is the coefficient of variation of a time-series. An asterisk indicates coefficients that were significant at P ≤ 0.05.
Extended Data Fig. 3 Animal time-series with linear, nonlinear, or non-predictable population dynamics.
a, Animal time-series arranged by taxonomic class. b, Animal time-series were arranged by taxonomic order where sample size ≥ 10. Bolded numbers show sample size.
Extended Data Fig. 4 Final model results of dimensionality (E).
PC1 is the first principal component of life history traits, representing a combination of body length (mm), minimum age of first reproduction (months), and longevity (months) of animals (positive coefficient estimates = faster life histories; Extended Data Fig. 1). N is the time-series length. An asterisk indicates coefficients that were significant at P ≤ 0.05.
Extended Data Fig. 5 Final model results of forecast skill (ρ).
PC1 is the first principal component of life history traits, representing a combination of body length (mm), minimum age of first reproduction (months), and longevity (months) of animals. Linear, nonlinear, and not predictable represent the categorization of population dynamics. CV is the coefficient of variation of a time-series. An asterisk indicates coefficients that were significant at P ≤ 0.05.
Extended Data Fig. 6 Repeating zeroes in datasets do not change likelihood of nonlinearity.
Proportion of linearity/nonlinearity in animal time-series, arranged by taxonomic class. Due to some time-series having long sequences of zeroes, we filtered out time-series with strings of zeroes. a, Time-series with no filtering. b, Strings of zeroes > 20 filtered. c, Strings of zeroes > 5 filtered. d, Strings of zeroes > 1 filtered.
Extended Data Fig. 7 Summary statistics for animal time-series by taxonomic class.
Median time-series length represents median number of time-series data for the final dataset. Predictable datasets were categorized if the Pearson correlation coefficient of out-of-sample prediction was significant at P ≤ 0.05.
Extended Data Fig. 8 Examples of predictable time-series.
a, Standardized abundance of woodcock (Scolopax minor) over time. b, Standardized abundance of grey red-backed voles (Myodes rufocanus) over time. c, Standardized abundance of dover soles (Solea solea) over time. d, Standardized abundance of woolly beech aphids (Phyllaphis fagi) over time. Grey lines represent the observed abundance, blue lines represent predicted abundance.
Supplementary information
Supplementary Information
Supplementary methods, Tables 1–3 and results.
Rights and permissions
About this article
Cite this article
Clark, T.J., Luis, A.D. Nonlinear population dynamics are ubiquitous in animals. Nat Ecol Evol 4, 75–81 (2020). https://doi.org/10.1038/s41559-019-1052-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41559-019-1052-6
This article is cited by
-
Interaction network structure explains species’ temporal persistence in empirical plant–pollinator communities
Nature Ecology & Evolution (2024)
-
The recovery of European freshwater biodiversity has come to a halt
Nature (2023)
-
Chaos is not rare in natural ecosystems
Nature Ecology & Evolution (2022)
-
The dynamical complexity of seasonal soundscapes is governed by fish chorusing
Communications Earth & Environment (2022)
-
Causal networks of phytoplankton diversity and biomass are modulated by environmental context
Nature Communications (2022)