Abstract
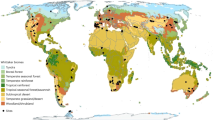
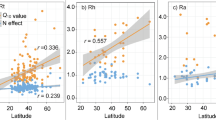
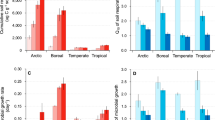
Soil respiration represents a major carbon flux between terrestrial ecosystems and the atmosphere, and is expected to accelerate under climate warming. Despite its importance in climate change forecasts, however, our understanding of the effects of temperature on soil respiration (RS) is incomplete. Using a metabolic ecology approach we link soil biota metabolism, community composition and heterotrophic activity to predict RS rates across five biomes. We find that accounting for the ecological mechanisms underpinning decomposition processes predicts climatological RS variations observed in an independent dataset (n = 312). The importance of community composition is evident because without it RS is substantially underestimated. With increasing temperature, we predict a latitudinal increase in RS temperature sensitivity, with Q10 values ranging between 2.33 ± 0.01 in tropical forests to 2.72 ± 0.03 in tundra. This global trend has been widely observed, but has not previously been linked to soil communities.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Hicks Pries, C. E., Castanha, C., Porras, R. C. & Torn, M. S. The whole-soil carbon flux in response to warming. Science 355, 1420–1423 (2017).
Davidson, E. A. & Janssens, I. A. Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 440, 165–173 (2006).
Crowther, T. W. et al. Quantifying global soil carbon losses in response to warming. Nature 540, 104–108 (2016).
Bradford, M. A. et al. Managing uncertainty in soil carbon feedbacks to climate change. Nat. Clim. Change 6, 751–758 (2016).
Bond-Lamberty, B. & Thomson, A. Temperature-associated increases in the global soil respiration record. Nature 464, 579–582 (2010).
Cox, P. M., Betts, R. A., Jones, C. D., Spall, S. A. & Totterdell, I. J. Acceleration of global warming due to carbon-cycle feedbacks in a coupled climate model. Nature 408, 184–187 (2000).
Koven, C. D., Hugelius, G., Lawrence, D. M. & Wieder, W. R. Higher climatological temperature sensitivity of soil carbon in cold than warm climates. Nat. Clim. Change 7, 817–822 (2017).
Giardina, C. P., Litton, C. M., Crow, S. E. & Asner, G. P. Warming-related increases in soil CO2 efflux are explained by increased below-ground carbon flux. Nat Clim. Change 4, 822–827 (2014).
Reichstein, M. et al. On the separation of net ecosystem exchange into assimilation and ecosystem respiration: review and improved algorithm. Glob. Change Biol. 11, 1424–1439 (2005).
Raich, J. W. & Schlesinger, W. H. The global carbon dioxide flux in soil respiration and its relationship to vegetation and climate. Tellus B 44, 81–99 (1992).
Exbrayat, J. F., Pitman, A. J., Zhang, Q., Abramowitz, G. & Wang, Y. P. Examining soil carbon uncertainty in a global model: response of microbial decomposition to temperature, moisture and nutrient limitation. Biogeosciences 10, 7095–7108 (2013).
Yang, J. et al. The role of satellite remote sensing in climate change studies. Nat. Clim. Change 3, 875–883 (2013).
Rustad, L. E. et al. A meta-analysis of the response of soil respiration, net nitrogen mineralization, and aboveground plant growth to experimental ecosystem warming. Oecologia 126, 543–562 (2001).
Balser, T. C. & Wixon, D. L. Investigating biological control over soil carbon temperature sensitivity. Glob. Change Biol 15, 2935–2949 (2009).
Thakur, M. P. et al. Reduced feeding activity of soil detritivores under warmer and drier conditions. Nat. Clim. Change 8, 75–78 (2018).
Eisenhauer, N., Cesarz, S., Koller, R., Worm, K. & Reich, P. B. Global change belowground: impacts of elevated CO2, nitrogen, and summer drought on soil food webs and biodiversity. Glob. Change Biol. 18, 435–447 (2012).
Suttle, K. B., Thomsen, M. A. & Power, M. E. Species interactions reverse grassland responses to changing climate. Science 315, 640–642 (2007).
Yvon-Durocher, G. et al. Reconciling the temperature dependence of respiration across timescales and ecosystem types. Nature 487, 472–476 (2012).
Ehnes, R. B., Rall, B. C. & Brose, U. Phylogenetic grouping, curvature and metabolic scaling in terrestrial invertebrates. Ecol. Lett. 14, 993–1000 (2011).
Briones, M. J. I., Ostle, N. J., McNamara, N. P. & Poskitt, J. Functional shifts of grassland soil communities in response to soil warming. Soil Biol. Biochem. 41, 315–322 (2009).
Sibly, R. M., Brown, J. H. & Kodric-Brown, A. Metabolic Ecology: A Scaling Approach (Wiley-Blackwell, Oxford, 2012).
Brown, J. H. & Sibly, R. M. in Metabolic Ecology (eds Sibly, R. M., Brown, J. H. & Kodric-Brown, A.) Ch. 2 (Wiley-Blackwell, Oxford, 2012).
Brown, J. H., Gillooly, J. F., Allen, A. P., Savage, V. M. & West, G. B. Toward a metabolic theory of ecology. Ecology 85, 1771–1789 (2004).
Kozlowski, J., Konarzewski, M. & Gawelczyk, A. T. Cell size as a link between noncoding DNA and metabolic rate scaling. Proc. Natl Acad. Sci. USA 100, 14080–14085 (2003).
Lampert, W. in A Manual on Methods for the Assessment of Secondary Productivity in Fresh Waters (ed. Edmonson, W. T.) 413–468 (Blackwell Scientific, Oxford, Edinburgh, 1984).
Peters, R. H. The Ecological Implications of Body Size. Vol. 2 (Cambridge Univ. Press, Cambridge, 1983).
Bond-Lamberty, B. & Thomson, A. A global database of soil respiration data. Biogeosciences 7, 1915–1926 (2010).
Karhu, K. et al. Temperature sensitivity of soil respiration rates enhanced by microbial community response. Nature 513, 81–84 (2014).
Clein, J. S. & Schimel, J. P. Microbial activity of tundra and taiga soils at sub-zero temperatures. Soil Biol. Biochem. 27, 1231–1234 (1995).
Dorrepaal, E. et al. Carbon respiration from subsurface peat accelerated by climate warming in the subarctic. Nature 460, 616–619 (2009).
Nie, M. et al. Positive climate feedbacks of soil microbial communities in a semi-arid grassland. Ecol. Lett. 16, 234–241 (2013).
Aerts, R. The freezer defrosting: global warming and litter decomposition rates in cold biomes. J. Ecol 94, 713–724 (2006).
Davidson, E. A., Janssens, I. A. & LuoY. On the variability of respiration in terrestrial ecosystems: moving beyond Q10. Glob. Change Biol. 12, 154–164 (2006).
Mikan, C. J., Schimel, J. P. & Doyle, A. P. Temperature controls of microbial respiration in arctic tundra soils above and below freezing. Soil Biol. Biochem. 34, 1785–1795 (2002).
Chen, H. & Tian, H. Q. Does a general temperature‐dependent Q10 model of soil respiration exist at biome and global scale? J. Integr. Plant Biol. 47, 1288–1302 (2005).
Mahecha, M. D. et al. Global convergence in the temperature sensitivity of respiration at ecosystem level. Science 329, 838–840 (2010).
Meehan, T. D. Mass and temperature dependence of metabolic rate in litter and soil invertebrates. Physiol. Biochem. Zool. 79, 878–884 (2006).
Chown, S. L. et al. Scaling of insect metabolic rate is inconsistent with the nutrient supply network model. Funct. Ecol. 21, 282–290 (2007).
Makarieva, A. M., Gorshkov, V. G. & LiB.-L. Energetics of the smallest: do bacteria breathe at the same rate as whales? Proc. R Soc. B 272, 2219–2224 (2005).
Laybourn, J. & Finlay, B. J. Respiratory energy losses related to cell weight and temperature in ciliated protozoa. Oecologia 24, 349–355 (1976).
Fenchel, T. & Finlay, B. J. Respiration rates in heterotrophic, free-living protozoa. Microb. Ecol. 9, 99–122 (1983).
Klekowski, R., Wasilewska, L. & Paplinska, E. Oxygen consumption by soil-inhabiting nematodes. Nematologica 18, 391–403 (1972).
Ferris, H., Lau, S. & Venette, R. Population energetics of bacterial-feeding nematodes: respiration and metabolic rates based on CO2 production. Soil Biol. Biochem. 27, 319–330 (1995).
Nielsen, C. O. Respiratory metabolism of some populations of enchytraeid worms and freeliving nematodes. Oikos 12, 17–35 (1961).
Fierer, N., Strickland, M. S., Liptzin, D., Bradford, M. A. & Cleveland, C. C. Global patterns in belowground communities. Ecol. Lett. 12, 1238–1249 (2009).
Xu, X., Thornton, P. E. & Post, W. M. A global analysis of soil microbial biomass carbon, nitrogen and phosphorus in terrestrial ecosystems. Glob. Ecol. Biogeogr. 22, 737–749 (2013).
Petersen, H. & Luxton, M. A comparative analysis of soil fauna populations and their role in decomposition processes. Oikos 39, 288–388 (1982).
Subke, J. A., Inglima, I. & Francesca Cotrufo, M. Trends and methodological impacts in soil CO2 efflux partitioning: a metaanalytical review. Glob. Change Biol. 12, 921–943 (2006).
Bond-Lamberty, B., Wang, C, & Gower, S. T. A global relationship between the heterotrophic and autotrophic components of soil respiration?. Glob. Change Biol. 10, 1756–1766 (2004).
Hogberg, P., Nordgren, A., Buchmann, N. & TaylorA. F. Large-scale forest girdling shows that current photosynthesis drives soil respiration. Nature 411, 789–792 (2001).
Carey, J. C. et al. Temperature response of soil respiration largely unaltered with experimental warming. Proc. Natl Acad. Sci. USA 113, 13797–13802 (2016).
Acknowledgements
This research has been financially supported by a Natural Environment Research Council Soil Security Programme fellowship (NE/N019504/1). We thank C. Venditti, J. Brown, G. Yvon-Durocher and C. Hall for their feedback and suggestions on the manuscript.
Author information
Authors and Affiliations
Contributions
A.S.A.J. conceived the idea and compiled and analysed the data. A.S.A.J. and R.M.S. developed the methodology and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Tables 1–5, Supplementary Figures 1–4, Supplementary References
Rights and permissions
About this article
Cite this article
Johnston, A.S.A., Sibly, R.M. The influence of soil communities on the temperature sensitivity of soil respiration. Nat Ecol Evol 2, 1597–1602 (2018). https://doi.org/10.1038/s41559-018-0648-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41559-018-0648-6