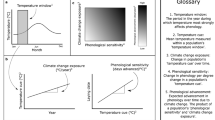
Abstract
Threats to species under climate change can be understood as a time at which the signal of climate change in ecological processes emerges from the noise of ecosystem variability, defined as ‘time of emergence’ (ToE). Here we show that ToE for the great tit (Parus major) will occur earlier at the level of population size than trait (laying date) and vital rates (survival, recruitment) under the RCP 8.5 scenario, suggesting an amplified climate change signal at the population level. ToE thus varies across levels of biological organization that filter trends and variability in climate differently. This has implications for the detection of climate impacts on wild species, as a shift in population size may precede changes in traits and vital rates. Further work would need to identify the ecological level that may experience an earlier detection of the climate signal for species with contrasting life histories, climate trends and variability.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
Data used in the analysis are available at https://github.com/marleng/ToE_greattit. Data on past observed beech crop index, past observed mismatch between laying dates and food peaks, expected spring temperature according to the RCP 8.5 scenario, past observed annual age-specific population size and vital rates and their variance are provided.
Code availability
R code used for the analysis is available at https://github.com/marleng/ToE_greattit.
References
Jenouvrier, S. et al. Detecting climate signals in populations across life histories. Glob. Change Biol. 28, 2236–2258 (2022).
Malhi, Y. et al. Climate change and ecosystems: threats, opportunities and solutions. Phil. Trans. R. Soc. B 375, 20190104 (2020).
Mahlstein, I., Knutti, R., Solomon, S. & Portmann, R. W. Early onset of significant local warming in low latitude countries. Environ. Res. Lett. 6, 034009 (2011).
Landrum, L. & Holland, M. M. Extremes become routine in an emerging new Arctic. Nat. Clim. Change 10, 1108–1115 (2020).
Rojas, M., Lambert, F., Ramirez-Villegas, J. & Challinor, A. J. Emergence of robust precipitation changes across crop production areas in the 21st century. Proc. Natl Acad. Sci. USA 116, 6673–6678 (2019).
Clements, C. F., Blanchard, J. L., Nash, K. L., Hindell, M. A. & Ozgul, A. Body size shifts and early warning signals precede the historic collapse of whale stocks. Nat. Ecol. Evol. 1, 0188 (2017).
Clements, C. F. & Ozgul, A. Including trait-based early warning signals helps predict population collapse. Nat. Commun. 7, 10984 (2016).
Baruah, G., Clements, C. F., Guillaume, F. & Ozgul, A. When do shifts in trait dynamics precede population declines? Am. Nat. 193, 633–644 (2019).
Hilde, C. H. et al. The demographic buffering hypothesis: evidence and challenges. Trends Ecol. Evol. 35, 523–538 (2020).
Visser, M. E., van Noordwijk, A. J., Tinbergen, J. M. & Lessells, C. M. Warmer springs lead to mistimed reproduction in great tits (Parus major). Proc. R. Soc. B 265, 1867–1870 (1998).
Reed, T. E., Jenouvrier, S. & Visser, M. E. Phenological mismatch strongly affects individual fitness but not population demography in a woodland passerine. J. Anim. Ecol. 82, 131–144 (2013).
Övergaard, R., Gemmel, P. & Karlsson, M. Effects of weather conditions on mast year frequency in beech (Fagus sylvatica L.) in Sweden. Int. J. For. Res. 80, 555–565 (2007).
Nussbaumer, A. et al. Patterns of mast fruiting of common beech, sessile and common oak, Norway spruce and Scots pine in Central and Northern Europe. For. Ecol. Manag. 363, 237–251 (2016).
Perdeck, A. C., Visser, M. E. & Van Balen, J. H. Great tit Parus major survival and the beech-crop cycle. Ardea 88, 99–106 (2000).
Kay, J. E. et al. The Community Earth System Model (CESM) large ensemble project: a community resource for studying climate change in the presence of internal climate variability. Bull. Am. Meteorol. Soc. 96, 1333–1349 (2015).
Visser, M. E., Lindner, M., Gienapp, P., Long, M. C. & Jenouvrier, S. Recent natural variability in global warming weakened phenological mismatch and selection on seasonal timing in great tits (Parus major). Proc. R. Soc. B 288, 20211337 (2021).
Both, C. & Visser, M. E. Adjustment to climate change is constrained by arrival date in a long-distance migrant bird. Nature 411, 296–298 (2001).
Porlier, M. et al. Variation in phenotypic plasticity and selection patterns in blue tit breeding time: between- and within-population comparisons. J. Anim. Ecol. 81, 1041–1051 (2012).
Gamelon, M. et al. Environmental drivers of varying selective optima in a small passerine: a multivariate, multiepisodic approach. Evolution 72, 2325–2342 (2018).
Marrot, P., Charmantier, A., Blondel, J. & Garant, D. Current spring warming as a driver of selection on reproductive timing in a wild passerine. J. Anim. Ecol. 87, 754–764 (2018).
Le Vaillant, J., Potti, J., Camacho, C., Canal, D. & Martínez-Padilla, J. Fluctuating selection driven by global and local climatic conditions leads to stasis in breeding time in a migratory bird. J. Evol. Biol. 34, 1541–1553 (2021).
Vatka, E., Orell, M., Rytkönen, S. & Merilä, J. Effects of ambient temperatures on evolutionary potential of reproductive timing in boreal passerines. J. Anim. Ecol. 90, 367–375 (2021).
Sæther, B.-E., Engen, S., Gustafsson, L., Grøtan, V. & Vriend, S. J. G. Density-dependent adaptive topography in a small passerine bird, the collared flycatcher. Am. Nat. 197, 93–110 (2021).
Charmantier, A. et al. Adaptive phenotypic plasticity in response to climate change in a wild bird population. Science 320, 800–804 (2008).
Matthysen, E., Adriaensen, F. & Dhondt, A. A. Multiple responses to increasing spring temperatures in the breeding cycle of blue and great tits (Cyanistes caeruleus, Parus major). Glob. Change Biol. 17, 1–16 (2011).
Charmantier, A. & Gienapp, P. Climate change and timing of avian breeding and migration: evolutionary versus plastic changes. Evol. Appl. 7, 15–28 (2014).
de Villemereuil, P. et al. Fluctuating optimum and temporally variable selection on breeding date in birds and mammals. Proc. Natl Acad. Sci. USA 117, 31969–31978 (2020).
Besbeas, P., Freeman, S. N., Morgan, B. J. T. & Catchpole, E. A. Integrating mark–recapture–recovery and census data to estimate animal abundance and demographic parameters. Biometrics 58, 540–547 (2002).
Schaub, M. & Abadi, F. Integrated population models: a novel analysis framework for deeper insights into population dynamics. J. Ornithol. 152, 227–237 (2011).
Brooks, S. P., King, R. & Morgan, B. J. T. A Bayesian approach to combining animal abundance and demographic data. Anim. Biodivers. Conserv. 27, 515–529 (2004).
Schaub, M. & Kéry, M. Integrated Population Models: Theory and Ecological Applications with R and JAGS (Elsevier Science, 2021).
Drobyshev, I., Niklasson, M., Mazerolle, M. J. & Bergeron, Y. Reconstruction of a 253-year long mast record of European beech reveals its association with large scale temperature variability and no long-term trend in mast frequencies. Agric. For. Meteorol. 192–193, 9–17 (2014).
Bogdziewicz, M., Kelly, D., Thomas, P. A., Lageard, J. G. A. & Hacket-Pain, A. Climate warming disrupts mast seeding and its fitness benefits in European beech. Nat. Plants 6, 88–94 (2020).
Bogdziewicz, M. et al. Climate warming causes mast seeding to break down by reducing sensitivity to weather cues. Glob. Change Biol. 27, 1952–1961 (2021).
Reed, T. E., Grøtan, V., Jenouvrier, S., Sæther, B.-E. & Visser, M. E. Population growth in a wild bird is buffered against phenological mismatch. Science 340, 488–491 (2013).
Beaumont, L. J. et al. Impacts of climate change on the world’s most exceptional ecoregions. Proc. Natl Acad. Sci. USA 108, 2306–2311 (2011).
Hawkins, E. et al. Observed emergence of the climate change signal: from the familiar to the unknown. Geophys. Res. Lett. 47, e2019GL086259 (2020).
Husby, A., Kruuk, L. E. B. & Visser, M. E. Decline in the frequency and benefits of multiple brooding in great tits as a consequence of a changing environment. Proc. R. Soc. B 276, 1845–1854 (2009).
Grøtan, V. et al. Spatial and temporal variation in the relative contribution of density dependence, climate variation and migration to fluctuations in the size of great tit populations. J. Anim. Ecol. 78, 447–459 (2009).
Dhondt, A. A., Adriaensen, F., Matthysen, E. & Kempenaers, B. Nonadaptive clutch sizes in tits. Nature 348, 723–725 (1990).
Ramakers, J. J. C., Gienapp, P. & Visser, M. E. Comparing two measures of phenological synchrony in a predator–prey interaction: simpler works better. J. Anim. Ecol. 89, 745–756 (2020).
Schwalm, C. R., Glendon, S. & Duffy, P. B. RCP8.5 tracks cumulative CO2 emissions. Proc. Natl Acad. Sci. USA 117, 19656–19657 (2020).
Kéry, M. & Schaub, M. Bayesian Population Analysis Using WinBUGS: A Hierarchical Perspective (Academic Press, 2012).
Lebreton, J.-D. & Gimenez, O. Detecting and estimating density dependence in wildlife populations. J. Wildl. Manage. 77, 12–23 (2013).
Lande, R. et al. Estimating density dependence from population time series using demographic theory and life-history data. Am. Nat. 159, 321–337 (2002).
Lebreton, J.-D., Burnham, K. P., Clobert, J. & Anderson, D. R. Modeling survival and testing biological hypotheses using marked animals: a unified approach with case studies. Ecol. Monogr. 62, 67–118 (1992).
de Valpine, P. & Hastings, A. Fitting population models incorporating process noise and observation error. Ecol. Monogr. 72, 57–76 (2002).
de Valpine, P. et al. Programming with models: writing statistical algorithms for general model structures with NIMBLE. J. Comput. Graph. Stat. 26, 403–413 (2017).
Brooks, S. P. & Gelman, A. General methods for monitoring convergence of iterative simulations. J. Comput. Graph. Stat. 7, 434–455 (1998).
Gamelon, M. et al. Density dependence in an age-structured population of great tits: identifying the critical age classes. Ecology 97, 2479–2490 (2016).
Hansen, B. B. et al. More frequent extreme climate events stabilize reindeer population dynamics. Nat. Commun. 10, 1616 (2019).
Abadi, F. et al. Estimating the strength of density dependence in the presence of observation errors using integrated population models. Ecol. Modell. 242, 1–9 (2012).
Gamelon, M. et al. Interactions between demography and environmental effects are important determinants of population dynamics. Sci. Adv. 3, e1602298 (2017).
Freckleton, R. P., Watkinson, A. R., Green, R. E. & Sutherland, W. J. Census error and the detection of density dependence. J. Anim. Ecol. 75, 837–851 (2006).
Schaub, M., Jakober, H. & Stauber, W. Strong contribution of immigration to local population regulation: evidence from a migratory passerine. Ecology 94, 1828–1838 (2013).
R Development Core Team R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2017).
Acknowledgements
We are grateful to all those who have collected data and to L. Vernooij and J. Risse for maintaining the long-term great tit database. We thank the board of the National Park of Hoge Veluwe for permission to carry out our research in their park. This work was supported by the Research Council of Norway through its Centres of Excellence funding scheme, project number 223257, by NASA grant number 80NSSC20K1289 to S.J. and by the CNRS.
Author information
Authors and Affiliations
Contributions
All the authors conceived the research idea. M.G. and S.J. designed the methodology and carried out the formal analysis. M.E.V. undertook data acquisition and M.E.V. and B.-E.S. funding acquisition. M.G. wrote the original draft and created the visuals and all authors were involved with reviewing and editing the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Climate Change thanks Qing Zhao and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Forecast of food peak dates, laying dates and mismatch under the RCP 8.5 scenario in the studied population between 1920 and 2100, when all sources of uncertainties are accounted for in the projections.
Black line corresponds to the median, shaded dark gray corresponds to 66% and light gray to 95% prediction intervals. Vertical dotted lines indicate the historical period (1922–1950), horizontal line indicates the lower bound of the 66% interval during that period for food peak dates and laying dates and indicates the upper bound of the interval for the mismatch. Vertical red lines correspond to the time of emergence (ToE). In yellow, annual observed values between 1985 and 2020 are provided.
Extended Data Fig. 2 Directed acyclic graph of the integrated population model (IPM). Squares represent the data, circles represent the parameters to be estimated.
Arrows represent dependencies. Three types of demographic data are collected: CMR data (m), count data (C) and fecundity data (number of daughters locally recruited (J) and number of mothers (B) in each age class i at year t). Parameters estimated with the IPM are the annual recapture probability pt, annual age-specific survival Si,t, annual age-specific recruitment Ri,t, annual number of immigrants Nimt and annual population size Nt.
Extended Data Fig. 3 Two extreme scenarios of beech crop production (scenarios 1 and 2).
Displayed are the probabilities of having a year of high BCI (level 3)). Black lines correspond to the median, shaded dark gray corresponds to 66% and light gray to 95% prediction intervals.
Extended Data Fig. 4 Age-specific vital rates, number of immigrants and population size forecasted in the studied great tit population between 1920 and 2100, when climate uncertainty is accounted for in the projections, under a scenario of decreasing beech crop production (scenario 1).
Each line corresponds to one climate scenario (40 in total), and the black lines correspond to the mean. Vertical dotted lines indicate the historical period (1922–1950), horizontal lines indicate the lower bound of the 66% interval during that period. Vertical red lines correspond to the time of emergence (ToE).
Extended Data Fig. 5 Age-specific vital rates, number of immigrants and population size forecasted in the studied great tit population between 1920 and 2100, when all sources of ecological uncertainties are accounted for in the projections, under a scenario of decreasing beech crop production (scenario 1).
Black lines correspond to the median, shaded dark gray corresponds to 66% and light gray to 95% prediction intervals. Vertical dotted lines indicate the historical period (1922–1950), horizontal line indicates the lower bound of the 66% interval during that period. Vertical red line corresponds to the time of emergence (ToE).
Extended Data Fig. 6 Age-specific vital rates, number of immigrants and population size forecasted in the studied great tit population between 1920 and 2100, when climate uncertainty is accounted for in the projections, under a scenario of increasing beech crop production (scenario 2).
Each line corresponds to one climate scenario (40 in total), and the black lines correspond to the mean. Vertical dotted lines indicate the historical period (1922–1950), horizontal lines indicate the upper bound of the 66% interval during that period. Vertical red lines correspond to the time of emergence (ToE).
Extended Data Fig. 7 Age-specific vital rates, number of immigrants and population size forecasted in the studied great tit population between 1920 and 2100, when all sources of ecological uncertainties are accounted for in the projections, under a scenario of increasing beech crop production (scenario 2).
Black lines correspond to the median, shaded dark gray corresponds to 66% and light gray to 95% prediction intervals. Vertical dotted lines indicate the historical period (1922–1950), horizontal line indicates the upper bound of the 66% interval during that period. Vertical red line corresponds to the time of emergence (ToE).
Supplementary information
Supplementary Information
Supplementary Fig. 1 and Table 1.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gamelon, M., Jenouvrier, S., Lindner, M. et al. Detecting climate signals cascading through levels of biological organization. Nat. Clim. Chang. 13, 985–989 (2023). https://doi.org/10.1038/s41558-023-01760-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41558-023-01760-y