Abstract
Alterations of marine species’ ranges with climate change are often attributed to oxygen limitation in warming oceans. Here we report unique metabolic temperature sensitivities for the myriad of vertically migrating oceanic species that daily cross depth-related gradients in temperature and oxygen. In these taxa, selection favours high metabolic activity for predator–prey interactions in warm shallow water and hypoxia tolerance in the cold at depth. These diverging selective pressures result in thermal insensitivity of oxygen supply capacity and enhanced thermal sensitivity of active metabolic rate. Aerobic scope is diminished in the cold, well beyond thermodynamic influences and regardless of ambient oxygen levels, explaining the native distributions of tropical migrators and their recent range expansions following warming events. Cold waters currently constitute an energetic barrier to latitudinal range expansion in vertical migrators. As warming due to climate change approaches, and eventually surpasses, temperatures seen during past warming events, this energetic barrier will be relieved.
Similar content being viewed by others
Main
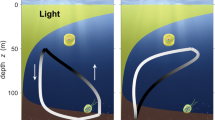
A myriad of oceanic species, from diminutive krill to jumbo squids, descend to depth in the daytime, seeking refuge from predators, and return to surface waters at night to feed. These synchronous daily movements may be the largest animal migration, and the midwater environment they traverse is potentially the largest ecosystem on the planet1. Vertical migrators are critical components of pelagic ecosystems worldwide, especially in regions with pronounced oxygen minimum zones (OMZ)2. Their metabolism and vertical displacements contribute substantially to elemental fluxes from surface to depth and to oxygen depletion in the OMZ3,4,5,6. Although this migration spans only a few hundred metres, it often crosses strong, correlated gradients in light, temperature and oxygen. Warm surface temperatures impose physiological stress on some vertical migrators7,8,9, while extreme hypoxia at depth triggers metabolic suppression and constrains habitable space in others10,11,12. Thus, compression between climate change-induced warming surface waters, where light renders zooplankton more vulnerable to predation, and de-oxygenating deeper waters is a growing concern13,14,15,16,17.
Latitudinal range shifts in marine animals are commonly linked to climate warming18. However, ranges for some species contract while others expand and the rates of population movement often differ at the leading and trailing edges19. The precise mechanisms imposing latitudinal range shifts are uncertain, especially for oceanic species, including vertical migrators. In recent decades, the abundance of tropical zooplankton increased in North Pacific temperate waters during warming events (for example, El Niño and warm-water anomalies20,21,22. At the same time, the jumbo squid, Dosidicus gigas, expanded its tropical range into temperate waters23. The tropicalization of the temperate community that resulted from range expansion of zooplankton and the squid had cascading effects throughout food webs23,24. Latitudinal range shifts for vertical migrators have been attributed to a combination of productivity changes, ecological and life-history influences and altered circulation patterns and chemical and physical changes in the environment20,25. However, the role of temperature is often dismissed because migrators appear to tolerate a broad temperature range between their day and night habitat23. Regardless, their distributions and contributions to biogeochemical cycles are a function of the temperature sensitivities of the underlying physiological traits, which remain poorly described26.
Deep, dark tropical waters convey distinct selective pressures from shallow well-lit temperate waters, despite similarly cold temperatures. We argue that these differences play a key role in the temperature sensitivity of metabolic capacity with consequences for latitudinal range limits. Using the Metabolic Index framework27,28,29 (Fig. 1), we show that for vertical migrators (oceanic species with distinct day and night depth ranges), cold, rather than warm, waters impose an energetic barrier to their distributions. This cold-water barrier results from strong selection for predator–prey interactions in warm surface waters and the relaxation of that selection at cold, mesopelagic depths30,31. This ecological reality is cemented into the migrator’s physiological capacity to supply oxygen, which must be high to support high oxygen demands near the surface and must remain high at depth, despite much lower oxygen demands, to tolerate the extreme hypoxia in the OMZ. Thus, in migrators, oxygen supply capacity is high and invariant across the depth and temperature range (Fig. 2). Here we used laboratory-derived temperature sensitivities of metabolic traits and recently derived relationships between them28 to map aerobic scope for several dominant vertical migrators, including euphausiids (krill) and the jumbo squid, Dosidicus gigas, inferring energetic constraints and opportunities that influence current and future habitat.
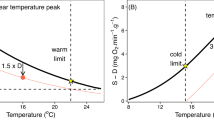
Maximum (MMR) and standard (SMR) metabolic rate, their critical oxygen pressures (Pcmax and PcSMR), factorial aerobic scope (FAS) and the oxygen supply capacity (α, the slope of the relationship between MR and PO2) change with temperature and oxygen in several ways. (1) MMR and α increase with temperature while the Pcmax is constant. (2) The α is insensitive to temperature and Pcmax changes in proportion to MMR. (3) Adaptation to low oxygen increases α and preserves FAS.
a–c, Standard metabolic rates (Y, SMR; a), critical oxygen pressures (Y, PcSMR; b) and the oxygen supply capacity (Y, α = SMR PcSMR−1; c) as a function of temperature (X) in tropical vertical migrators (red) and diverse coastal species (black; Supplementary Table 2). d, The temperature sensitivities (E) for SMR (X) and PcSMR (Y) are similar to each other (y = 0.94X + 0.21, n = 7 species; P = 0.06, two-tailed t-test; Supplementary Table 2), falling just above the dashed unity line. For coastal species, the SMR is more temperature sensitive than PcSMR (Supplementary Table 2; y = 0.62X - 0.06, n = 20 species; P = 4.3 × 10−5, two-tailed t-test). Thus, in migrators, the oxygen supply capacity is higher (P = 1.76 × 10−9; two-tailed t-test) and relatively insensitive to temperature (P = 3.06 × 10−10, two-tailed t-test) compared with coastal marine animals.
For a population to sustain itself, oxygen supply must meet demand. Oxygen demand is the metabolic rate (MR), measured as a rate of oxygen consumption. Oxygen supply is the product of environmental availability (PO2) and the physiological capacity to extract oxygen from the environment and to transport it to respiring tissues (α, the physiological oxygen supply capacity per unit time, mass and PO2; μmol g−1 h−1 kPa−1). The α depends on the respiratory (that is, gill) surface area, blood-oxygen binding and cardiac output among other traits. As environmental PO2 declines and/or as metabolic rate increases, physiological oxygen supply increases towards its capacity, α, which is quantifiable as the ratio of metabolic rate and the critical PO2 for that rate (Pc, the lowest PO2 that can sustain a given metabolic rate; Fig. 1, Supplementary Figs. 1 and 2 and equation (1)28,32). The α can be viewed from two equivalent perspectives: (1) it determines the lowest environmental PO2 that can support a given metabolic rate (that is, Pc) and (2) it determines the maximum metabolic rate (MMR) achievable at a given environmental PO2. Metabolism cannot increase with oxygen indefinitely, however, and above a certain environmental PO2, the ability to both supply and utilize oxygen are maximized and MR cannot further increase. This limit is Pcmax (the Pc for MMR). Oxygen supply has evolved such that Pcmax approximates the lowest PO2 that an organism persistently (longer than a diel or tidal cycle) encounters at a given temperature23. Thus, for most species measured to date, Pcmax is near air saturation (21 kPa) (ref. 28), and any decline in PO2 will result in a decrement in metabolic capacity.
The MR is typically measured either at rest in a fasted state (standard, SMR) or under maximum exertion (MMR), and aerobic scope is the factorial difference between these two rates (factorial aerobic scope, FAS; equation (2) and Fig. 1). A FAS greater than 1 permits metabolic rate to increase by that factor above SMR, and environmental PO2 must exceed the Pc at that rate (PcSMR) by that same factor to ensure sufficient oxygen availability. While FAS ranges between 1 and ~7 in acute temperature trials, a critical FAS value of ~3 is coincident with warm (Equatorward) range boundaries for a variety of species, providing a benchmark for metabolically available habitat29.
The temperature sensitivity of metabolic traits is described here by the slope of an Arrhenius function (E, the temperature coefficient; Supplementary Information). Because the metabolic traits are related to each other, the temperature coefficients are also related (equation (3) and Extended Data Fig. 1a)28,29.
Metabolic traits and their temperature sensitivity
We report SMR, PcSMR and α at temperatures approximating day and night habitats for six vertically migrating euphausiids (krill) and for the jumbo squid, Dosidicus gigas (Supplementary Tables 1 and 2 and Fig. 2). For D. gigas, the compiled data include MMR (Supplementary Table 1). We further compile metabolic traits for a diversity of coastal marine animals (Supplementary Table 2). Across this diversity of species, α, the physiological capacity to extract oxygen from the environment and transport it to respiring tissues, spans two orders of magnitude. Among coastal species, the PcSMR is much less variable than SMR between species or across temperatures (Fig. 2). Thus, most of the interspecific variability in α (SMR:PcSMR) is accounted for by differences in metabolic rate, whether driven by temperature and size effects or by ecological differences (that is, activity)28,29. However, specific adaptation to low oxygen among mesopelagic species in oxygen minimum zones, such as the vertical migrators studied here, also drives an increase in α (refs. 12,33). Across the measured temperature range, the mean α for the Eastern Pacific vertical migrators (3.84 µmol O2 g−1 h−1 kPa−1; Supplementary Table 1) is much higher than that for coastal marine species (0.64 ± 0.45 µmol O2 g−1 h−1 kPa−1).
For coastal species, the Pcmax is typically near air saturation and is independent of temperature28. Furthermore, the SMR is more temperature sensitive than PcSMR (Supplementary Table 2), such that α increases with temperature with a similar coefficient to MMR (mean Eα = 0.31 eV, n = 20 species; mean EMMR = 0.27, n = 7 species (ref. 28)). Thus, in coastal species, as temperature increases, SMR approaches MMR, PcSMR approaches the Pcmax and FAS declines towards 1, where the scope for activity beyond basic maintenance is nil. In these coastal species, the similar temperature coefficients for α and MMR suggest that oxygen supply evolves within air-saturated waters to match maximum oxygen demand across the temperature range28.
The thermal sensitivity of tropical migrators is fundamentally different. The PcSMR in the tropical migrators studied here is highly temperature sensitive with a coefficient equal to or greater than SMR. Thus, α (= SMR PcSMR−1) is relatively insensitive to temperature. In fact, α actually decreases slightly from 10 °C and 20 °C in all migrators (mean Eα = −0.21 eV, n = 7 species; Supplementary Table 2 and Fig. 2b). The relative temperature insensitivity means that the temperature coefficients for MMR and Pcmax, regardless of their absolute values, must be similar to each other (equation (3)). Thus, as MMR decreases with temperature, a lower environmental oxygen pressure is sufficient to meet that demand. If, due to a high temperature coefficient, Pcmax drops below ambient PO2 at any depth, then MMR and FAS will depend only on temperature, and oxygen has no effect. In contrast, if Pcmax remains higher than environmental PO2 at depth, then MMR and FAS will be oxygen limited.
Among migrators, the temperature sensitivity of MMR has been measured only in D. gigas. In this species, MMR does not scale in a typical exponential temperature-dependent manner. Instead, MMR is nearly insensitive to temperature between 10 °C and 20 °C (EMMR = 0.21) and extremely temperature sensitive between 20 °C and 25 °C (EMMR = 1.65; Extended Data Fig. 1). As a result, FAS increases dramatically in warm, shallow water and is very low at depth due to cold, regardless of ambient oxygen levels. In migrators, a high oxygen supply capacity in warm water supports high activity during migration to shallow, oxygenated water at night, while some residual aerobic capacity remains at cold depths in the pronounced OMZ.
Modelling aerobic scope and metabolically available habitat
To calculate FAS across latitudinal and depth gradients, we extracted temperature and dissolved oxygen (World Ocean Atlas 201834,35) for a geographic band 500 km to 2,000 km off the western coast of the Americas between 55° S and 55° N and from 0 m to 500 m depth. Below the surface mixed layer, the oxygen and temperature gradients vary with latitude (Figs. 3aand 4a,b), being most pronounced in tropical OMZs36. We also modelled FAS across a latitudinal gradient using sea surface temperature data for the 1997–1998 El Niño and for Coupled Model Intercomparison Project (CMIP) 6 Shared Socioeconomic Pathway (SSP) 5–8.5 climate projections for the years 2021–2040, 2041–2060 and 2081–2100.
a, Representative oxygen and temperature profiles in the upper 500 m depth in the Gulf of California (red) and the California Current System (blue) were used to calculate Pcmax. b, The difference between PO2 and Pcmax indicates depths at which MMR is increasingly oxygen limited (negative values, blue shading), which only occurs in the tropics (Gulf of California) and only if the temperature coefficient is less than 1.0 eV (dashed line indicates Pcmax calculated with a temperature coefficient of 0.3 eV, which is near the mean for MMR).
a,b, Seawater temperature (a) and oxygen (b) profiles from 500 km to 2,000 km off the coast of the eastern Pacific Ocean (Extended Data Fig. 4; World Ocean Atlas 2018). c, Colour map of Dosidicus gigas FAS calculated using measured rates and temperature coefficients (Supplementary Table 1 and Extended Data Fig. 1) and environmental profiles (a,b). d, FAS of the euphausiid, Nyctiphanes simplex, estimated with a high (EMMR = 1.0 eV) temperature coefficient for MMR. Calculations are anchored at 25 °C assuming that Pcmax is 21 kPa (air saturation) at that temperature. Grey shading indicates FAS < 1.
Throughout the native range for tropical diel migrators, surface waters are characterized by temperatures >25 °C and air-saturated waters (Fig. 4a,b). Using this as a starting point, we calculated MMR at 25 °C (from equation (2)), assuming Pcmax is air saturation (21 kPa) at that temperature, as has been shown in D. gigas. At 25 °C, the measured MMR for D. gigas was very similar to that calculated for air-saturated waters at that temperature based on equations (1)–(3) (ref. 37) (Supplementary Table 2). The calculated Pcmax very closely matches the environmental PO2 across the depth range in the squid’s native habitat (Fig. 3b) despite being calculated from metabolic traits measured in air-saturated water. For euphausiids, the calculated MMR resulted in FAS ranging from 4 to 6, which is at the upper end of the range measured for a diversity of species28,38,39, supporting the approach.
From the 25 °C and 21 kPa starting points, we modelled FAS using measured temperature coefficients for SMR and PcSMR. FAS was calculated for every latitudinal and depth bin. For D. gigas, we used measured temperature coefficients for MMR while, for euphausiids, we used a continuous range of possible coefficients to assess their effects on modelled FAS (Fig. 3 and Supplementary Fig. 3d). Within the native tropical range, any temperature coefficient (EMMR) less than ~1.0 eV resulted in modelled Pcmax values that were higher than the ambient PO2 for all migrators at all depths below the shallow mixed layer (Fig. 3a and Supplementary Fig. 3d). In that case, MMR would be oxygen limited, declining in proportion to PO2. In contrast, higher temperature coefficients (EMMR > 1.0 eV), such as that measured for D. gigas, result in a Pcmax below the ambient PO2 across the inhabited depth range (Fig. 3a). The MMR is not oxygen limited in that scenario, but it declines with depth due to temperature to values that are as low or lower at all depths than they would be if they were oxygen limited (Figs. 4c,d and 5). Thus, FAS for these tropical migrators must decline with depth at least as fast as ambient PO2 regardless of its temperature sensitivity.
a, Dosidicus gigas FAS in surface waters plotted for the current condition (thick black line) and for the 1997–1998 El Niño (orange to green coloured lines). Dashed lines indicate extrapolation beyond the measured temperature range (10–25 °C). b, D. gigas FAS in surface waters plotted for the current condition (thick black line) and for near, medium and long-term projections of anticipated sea-surface temperatures (SST) from the CMIP6 SSP5–8.5 model.
For all migrators studied, FAS currently reaches values greater than 3 (the benchmark for population viability based on warm boundaries in marine species) only in the upper ~200 m of the water column, coincident with the known nighttime distribution for most migrators, throughout their native range (~20° N to 20° S; Fig. 4c,d and Supplementary Fig. 4). FAS declines below 1 at deeper daytime depths (200–400 m) in some regions. Survival at such depths is achieved via metabolic suppression which reduces total energy demand by 50–80% relative to SMR10,11,40,41. The prevalence of daytime metabolic suppression among migrators supports our argument that high metabolic rates are not required at depth.
FAS in surface waters declined below 2–3 at the edge of their native latitudinal range, a pattern that was robust across a 95% confidence band of Monte Carlo simulations of the measured physiological traits for D. gigas (Extended Data Figs. 2 and 3). Higher latitudes are metabolically unavailable (FAS < 3) for D. gigas due to persistent cold and the squid’s high thermal sensitivity of MMR. For other migrators, the thermal sensitivity of MMR has not been directly measured. However, temperature coefficients for MMR less than 1.0 eV (Extended Data Fig. 1d) would suggest excess metabolic capacity at cold temperatures that could never be utilized in their native habitat due to oxygen limitation at depth. Thus, low-temperature sensitivity of MMR is unlikely to have evolved, consistent with the direct measurements in D. gigas. A coefficient greater than ~1.0 eV will nevertheless result in low aerobic scope across the entire depth range in temperate waters, acting as an energetic barrier to range expansion (Figs. 4–6 and Extended Data Fig. 4). Notably, FAS also increases with temperature in at least one euphausiid from the California Current42, suggesting that similar cold-water barriers to range expansion also exist for temperate migrators. However, our model shows that warmer waters, such as those experienced during the El Niño in 1997–1998 and that will be reached with climate change over the next several decades, are sufficient to expand metabolically available habitat by ~10–20° N and S (Fig. 5).
Representative regional oxygen pressure profiles are shown (dashed lines). Where Pcmax > PO2 in a given region, aerobic scope is oxygen limited (shading). Numbers and their placement on the plot indicate the FAS at that temperature, oxygen pressure and region. In the California Current (black line and shading), FAS is low due to temperature, and oxygen is not limiting until temperatures near 5 °C in the core of the OMZ there (~600 m depth). In the Gulf of California (light blue line and shading) oxygen becomes limiting only below 15 °C. In the Eastern Tropical Pacific (dark blue line and shading), oxygen is limiting at all depths, but aerobic scope is nevertheless high at high temperatures.
Analysis of climate change effects on the native habitat requires extrapolation beyond the measured temperature range and ignores the potential for other mechanisms to set critical temperatures. Noting those caveats, modelled aerobic scope does decline in the native tropical habitat as temperatures exceed 25 °C in surface waters due to a continual increase in oxygen demand while supply is constrained by atmospheric PO2 (Figs. 4c and 5). However, even under the climate change projections, FAS never declines to values near those currently achieved in temperate waters (~2.2; Fig. 5b).
Ocean de-oxygenation due to climate change10 would have to lower PO2 by more than 50% in temperate waters to limit FAS (Fig. 6). Even if oxygen were limiting (that is, if PO2 < Pcmax), FAS would decline by only 1 Pcmax−1 (that is ~5% per kPa PO2 in surface waters). Such small changes will have a negligible effect on these migrators in temperate waters and are unlikely to have much effect at any latitude because PO2 would have to decline beyond any reasonable projections to reduce FAS to values currently observed at cold temperatures (Fig. 6). Thus, in the context of de-oxygenation, the most likely effect of climate change is a modest compression of the native habitat between warming surface waters and the potentially expanding OMZ (Fig. 6b).
Discussion
Oceanic species that migrate daily across strong, persistent and correlated gradients in light, temperature and oxygen possess a unique metabolic temperature sensitivity. Our results suggest that the capacities for oxygen supply and demand are driven by ecological pressures rather than thermodynamic influences or environmental limitations. Low light levels at depth restrict visual predator–prey interactions, reduce selection for metabolic capacity31 and provide a daytime refuge from predation for vertical migrators43. Locomotory activities have been estimated for vertical migrators across the depth range using biologging44, acoustics45,46, in situ observations47,48 and laboratory measurements37,42. This mounting evidence suggests that most vertically migrating species are largely inactive at depth during the daytime. Selective pressure for metabolic capacity to support activity for predator–prey interactions in warm, shallow water and the absence of such selection at depth result in enhanced temperature sensitivity of active metabolism in vertical migrators.
At low temperature, aerobic scope is low, even when measured in air-saturated waters, because low temperature is experienced only at depth where high metabolic capacity is not required and has not evolved. Thus, temperature appears to act as a metabolic regulator beyond thermodynamic constraints. Physiological oxygen supply capacity is high in migrators at all temperatures, reflecting the extreme temperature sensitivity of the underlying oxygen transport mechanisms49,50 and supporting high activity in warm, aerated surface waters and the diminished oxygen requirements in the OMZ at depth.
We propose that the low aerobic scope in cold water precludes persistent occupation of temperate waters by tropical migrators. There, FAS is low across the typically inhabited depth range due to temperature while oxygen has no effect on tropical migrators in temperate waters (Figs. 3 and 4 and Extended Data Figs. 5–7). At these higher latitudes, FAS does not surpass 3, even at the surface, probably rendering this habitat metabolically unavailable (Fig. 4c,d and Extended Data Figs. 6 and 7). If so, the reported range expansions to high latitudes by squids23 and krill20 could occur only following warming events (for example, El Niño; Fig. 4a). Modest warming, such as that occurring during the 1997–1998 El Niño, elevated aerobic scope throughout temperate waters and expanded metabolically viable habitat by 10–20° N and S (Fig. 5). Ocean warming due to climate change may similarly expand metabolically available habitat at these higher latitudes, permanently altering oceanic ecosystems.
We have shown for tropical vertical migrators that (1) the oxygen supply capacity is high and relatively independent of temperature, (2) accordingly, MMR and its critical PO2 change with temperature in proportion to each other and (3) FAS declines with depth throughout the native habitat due to temperature. Low oxygen may further restrict FAS at depth in extreme OMZs, but high oxygen confers no benefit in cold water. These findings imply that temperature mediates metabolism in response to ecological demands for activity beyond simple thermodynamic effects on biochemical reaction rates.
Ocean warming is believed to cause a poleward drift in the thermal performance window for marine ectotherms and extirpation of animals from their native range51. Ocean de-oxygenation is predicted to compress available habitat, especially where the OMZ is shallow and intruding on the daytime habitat of vertical migrators52. In contrast to these predictions, we suggest that a modest increase in temperature, such as that associated with the 1997–1998 El Niño or projected with climate change (Fig. 5a), will result in a dramatic expansion of available habitat at higher latitudes for vertical migrators. While we lack measurements beyond 25 °C, our model predicts minimal loss of habitat in their native tropical range, despite ongoing warming and de-oxygenation (Fig. 6 and Extended Data Fig. 6). These opposing views predict different timing and extent of species’ distributional shifts due to climate change with consequences for the resulting community assemblages.
Methods
Data compilation
The metabolic traits (SMR, PcSMR, α) and their temperature coefficients (E) are derived from laboratory measurements at multiple temperatures. These metabolic traits are reported in Supplementary Table 1 (Dosidicus gigas, including maximum metabolic rate) and 2 (vertical migrators and coastal species). Measurements for four euphausiid species are reported here for the first time. All others are from published literature (Supplementary Table 2). Coastal species are those known to live predominantly in shallow, well-oxygenated waters over the continental shelf. Only crustaceans, cephalopods and fish are considered here as they possess reasonably comparable oxygen transport systems, inclusive of gills, respiratory proteins and at least partially closed circulatory systems. Except for D. gigas, all species’ metrics are reported only within a narrow body size range, insufficient for confident scaling analysis. Metabolic rates typically decline with body mass (M) according to MR = aMb, where a is a normalization constant and b is a scaling coefficient that describes the slope of the relationship. The scaling coefficients for metabolic traits are related to each other as in equation (4). If SMR declines with size but Pc does not, oxygen supply capacity will decline with size (equation (4) (ref. 28)). PcSMR is insensitive to body mass for D. gigas. However, b for SMR is also relatively shallow (b ~ −0.1; Extended Data Fig. 1c) compared with most species (b ~ 0.25). If Pcmax does not change with size, oxygen supply capacity will match MMR regardless of size (equation (4)). In any case, D. gigas metabolic rates were normalized to 100 g body mass using measured scaling coefficients (Extended Data Fig. 1c).
Animal capture
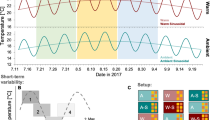
For four zooplankton species (euphausiids), live experiments were conducted according to ref. 12. Animals were collected using a modified Tucker trawl, which used standard MOCNESS control software and sensors and had a large insulated cod end to maintain species at their ambient (capture) temperatures when brought to the surface alive. A month-long research expedition from Manzanillo, Mexico, to San Diego, California, on the R/V Sikuliaq, cruise number SKQ201701S, occurred from 19 January to 15 February 2017 and was centred at 21.6° N, 117.8° W, an area with a strong OMZ.
Respirometry
Shipboard respiration measurements of key species (euphausiids) determined their oxygen supply25 at 10 °C and 20 °C. Following 6- to 12-hour acclimation at experimental temperature and air-saturated water, animals were placed in darkened sealed chambers filled with 0.2 µm filtered seawater treated with antibiotics (25 mg l−1 each of streptomycin and ampicillin) to minimize microbial respiration. Chamber size ranged from 2 ml to 50 ml, resulting in a ratio of chamber volume to animal mass of ~10 to 100. Seawater PO2 (oxygen partial pressure) was measured optically with a Loligo Systems Witrox 4 or PyroScience FireSting O2 meter. Animals were allowed to consume the ambient oxygen until the PO2 declined to a level insufficient to support their oxygen-consumption rate. Individual trial durations ranged from 6 hours to 48 hours. Temperature was maintained with Lauda E100 and Thermo Fisher Scientific NESLAB RTE-7 water baths. Oxygen meters were calibrated with air-saturated seawater and concentrated NaSO3 solution. Chambers were stirred with magnetic stirrers (Cole–Parmer Immersible Stirrer EW-04636-50). After the experiments were completed, animals were frozen at −80 °C before being weighed.
Oxygen supply capacity
Oxygen supply capacity was calculated as SMR Pc−1 for species with available published rates. For four euphausiid species, oxygen supply capacity was directly determined32. The MR, measured as above, was monitored as oxygen declined. Each trial was divided into discrete time bins to calculate multiple MR values over each trial. Bins of 1/10th the trial duration were used at the highest PO2 values (where precise rate measurement was a priority) and 1/100th the trial duration at the lowest PO2 values (where good PO2 resolution was a priority). For each measurement period, MR was divided by the corresponding PO2 to provide the concurrent oxygen provision (α0 = MR PO2−1). The average of the highest three α0 values was designated as α, the oxygen supply capacity. In each trial, the α is the slope of a line describing the rate dependence of Pc (Pc = MR α−1). The same value of α is reached at Pc for any MR regardless of previous or subsequent metabolic activity. Six representative trials, including three at each temperature, are provided for each of four species (Extended Data Figs. 7–10).
Mapping physiological parameters for all species
To calculate FAS plots, environmental data (temperature and dissolved oxygen) were pulled from the World Ocean Atlas 201834,35 for a geographic band 500 km to 2,000 km off the western coast of the Americas between 55° S and 55° N and across 37 depth bins from 0 m to 500 m depth. This range covered temperatures from 2.7 °C to 29 °C and oxygen levels from 0.1 kPa to 22.5 kPa. Measured SMR, PcSMR and α values and their temperature dependencies (E values) were drawn from Supplementary Table 2 for each species examined here. Then, these values were computed at any given temperature within the geographic range by the ‘adj_by_temp()’ function from the respirometry v. 1.4.0 R package53. For all species, Pcmax at 25 °C was assumed to be 21 kPa, because this is the average sea surface temperature in the eastern tropical Pacific, and marine animals that live in the mixed layer have a Pcmax near 21 kPa (ref. 28). MMR at 25 °C was estimated by 21 kPa × α25°C, and its temperature sensitivity (EMMR) was defined as either 0.3, which is the approximate mean value for coastal fish in Supplementary Table 2, or 1.0 eV, a value chosen as the minimum that avoids oxygen limitation (Extended Data Fig. 1d). EMMR has not been determined in any species examined here except Dosidicus gigas (below). Temperature-dependent MMR (MMRT) was defined for any given temperature based on this starting value and EMMR.
From these primary metrics, the following additional metrics were derived for every cell in the environmental array:
*using measured or modelled temperature coefficients for each metric.
The average of all observations at a given 0.25° latitude bin and depth (n ≈ 50–90 depending on latitude) was computed for all metrics.
Mapping physiological parameters for Dosidicus gigas
Mapping of physiological parameters to the environmental data was similar for Dosidicus gigas as for other species, except that MMR data (in addition to SMR) are available for this species and thus measured MMR and EMMR were used rather than computed metrics. Interestingly, MMR does not scale in a typical exponential temperature-dependent manner. Instead, MMR is quite temperature insensitive between 10 °C and 20 °C (EMMR = 0.21) and extremely temperature sensitive between 20 °C and 25 °C (EMMR = 1.65; Extended Data Fig. 1). Thus, when mapping FAS in relation to the environmental data, EMMR = 0.21 was used when T < 20 °C and EMMR = 1.65 was used when T ≥ 20 °C to most accurately align to the measured characteristics of the species.
Mapping Dosidicus gigas FAS during the 1997–1998 El Niño and climate change
Sea surface temperature was pulled from the National Oceanic and Atmospheric Administration’s (NOAA) Daily Optimum Interpolation Sea-Surface Temperature (DOISST) v2.1 dataset for the first day of each month from June 1997 through June 199854. The CMIP6 SSP5–8.5 projection for the years 2021–2040, 2041–2060 and 2081–2100 were similarly used to project sea surface temperature. Mapping of physiological metrics for Dosidicus gigas to these datasets was identical to that described above for the WOA data except that oxygen data were not available so were assumed to be 21 kPa at the surface. The FAS for the El Niño and climate change time series was then compared with the surface FAS values from the World Ocean Atlas (WOA) data.
Mapping model sensitivity analysis
A Monte Carlo simulation was run to determine the sensitivity of the mapping model to underlying variation in physiological measurements for the squid Dosidicus gigas (Extended Data Fig. 2). For each measured physiological trait (SMR, MMR and PcSMR), 5,000 draws were made from a normal distribution with mean and standard deviation matching the mean and standard error of that trait (Supplementary Table 1). In each iteration, the complete mapping model described above was simulated. The 2.5 and 97.5 percentiles of each derived trait (for example, FAS, Pcmax) at each latitude and depth bin across all 5,000 iterations were compiled, thus representing a 95% confidence interval for each spatial bin.
The results of this analysis indicate that while the absolute values may shift, the trend remains constant across the 95% confidence interval that FAS is ≥ 3 at the surface between about 15° S and 20° N and falls below 3 at higher latitudes (Supplementary Fig. 5a–c), matching well to the native range boundary for this species.
We also ran this sensitivity analysis with a ‘normal’ exponential temperature dependence (fixed E value across all measured temperatures) to ensure that the reliability of our model was not over-estimated in cold-water regions where the low-temperature dependence of metabolism may underestimate variability. In nearly all latitude and depth bins, the use of fixed E values for metabolic rates would have led to confidence intervals that were nearly the same or smaller than the model using changing E values to best fit the observed data. The code used in our analysis is available on Github47.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
World Ocean Atlas temperature and oxygen data (woa18_decav_t00_04.nc and woa18_all_O00_01.nc, respectively) are available at https://www.ncei.noaa.gov/access/world-ocean-atlas-2018/. CMIP6 climate projections are available at https://interactive-atlas.ipcc.ch/39965d78-e97e-42af-b488-b1e1f4d32f75. NOAA’s DOISST v2.1 dataset is available at https://www.ncei.noaa.gov/data/sea-surface-temperature-optimum-interpolation/v2.1/access/avhrr/.
Code availability
All code used in the production of this manuscript are available at
https://github.com/matthewabirk/DVM_temp_sensitivity (https://zenodo.org/badge/latestdoi/506078504).
References
Robison, B. H. Conservation of deep pelagic biodiversity. Conserv. Biol. 23, 847–858 (2009).
Fernandez-Alamo, M. A. & Färber-Lorda, J. Zooplankton and the oceanography of the eastern tropical Pacific: a review. Prog. Oceanogr. 69, 318–359 (2006).
Bianchi, D., Galbraith, E. D., Carozza, D. A., Mislan, K. A. S. & Stock, C. A. Intensification of open-ocean oxygen depletion by vertically migrating animals. Nat. Geosci. 6, 545–548 (2013).
Steinberg, D. K. & Landry, M. R. Zooplankton and the ocean carbon cycle. Annu. Rev. Mar. Sci. 9, 413–444 (2017).
Kiko, R. & Hauss, H. On the estimation of zooplankton-mediated active fluxes in oxygen minimum zones regions. Front. Mar. Sci. https://doi.org/10.3389/fmars.2019.00741 (2019).
Longhurst, A., Bedo, A., Harrison, W., Head, E. & Sameoto, D. Vertical flux of respiratory carbon by oceanic diel migrant biota. Deep Sea Res. Part I 37, 685–694 (1990).
Elder, L. E. & Seibel, B. A. The thermal stress response to diel vertical migration in the hyperiid amphipod, Phronima sedentaria. Comp. Biochem. Physiol. A 187, 20–26 (2015).
Tremblay, N., Gomez-Gutierrez, J., Zenteno-Savin, T., Robinson, C. J. & Sanchez-Velascoa, L. Role of oxidative stress in seasonal and daily vertical migration of three krill species in the Gulf of California. Limnol. Oceanogr. 55, 2570–2584 (2010).
Lopes, A. R. et al. Oxidative stress in deep scattering layers: heat shock response and antioxidant enzymes activities of myctophid fishes thriving in oxygen minimum zones. Deep Sea Res. Part I 82, 10–16 (2013).
Seibel, B. A., Schneider, J., Kaartvedt, S., Wishner, K. F. & Daly, K. L. Hypoxia tolerance and metabolic suppression in oxygen minimum zone euphausiids: implications for ocean deoxygenation and biogeochemical cycles. Integr. Comp. Biol. https://doi.org/10.1093/icb/icw091 (2016).
Seibel, B. A. et al. Metabolic suppression during protracted exposure to hypoxia in the jumbo squid, Dosidicus gigas, living in an oxygen minimum zone. J. Exp. Biol. 217, 2710–2716 (2014).
Wishner, K. F. et al. Ocean deoxygenation and zooplankton: very small oxygen differences matter. Sci. Adv. 4, eaau5180 (2018).
Koslow, J. A., Goericke, R., Lara-Lopez, A. & Watson, W. Impact of declining intermediate-water oxygen on deepwater fishes in the California Current. Mar. Ecol. Prog. Ser. 436, 207–218 (2011).
Oschlies, A. A committed fourfold increase in ocean oxygen loss. Nat. Commun. 12, 2307 (2021).
Wishner, K. F., Seibel, B. A. & Outram, D. Ocean deoxygenation and copepods: coping with oxygen minimum zone variability. Biogeosciences 17, 2315–2339 (2020).
Stramma, L. et al. Expansion of oxygen minimum zones may reduce available habitat for tropical pelagic fishes. Nat. Clim. Change 2, 33–37 (2012).
Köhn, E. E., Münnich, M., Vogt, M., Desmmet, F. & Gruber, N. Strong habitat compression by extreme shoaling events of hypoxic waters in the Eastern Pacific. J. Geophys. Res. Oceans 127, e2022JC018429 (2022).
Poloczanska, E. S. et al. Global imprint of climate change on marine life. Nat. Clim. Change 3, 919–925 (2013).
Pinsky, M. L., Selden, R. L. & Kitchel, Z. J. Climate-driven shifts in marine species ranges: scaling from organisms to communities. Annu. Rev. Mar. Sci. 12, 153–179 (2020).
Cavole, L. M. et al. Biological impacts of the 2013–2015 warm-water anomaly in the northeast Pacific: winners, losers, and the future. Oceanography 29, 273–285 (2016).
Lavaniegosa, B. E., Jiménez-Herrera, M. A. & Ambriz-Arreola, I. Unusually low euphausiid biomass during the warm years of 2014–2016 in the transition zone of the California Current. Deep Sea Res. Part II 1, 69–170 (2019).
Lilly, L. E. & Ohman, M. D. Euphausiid spatial displacements and habitat shifts in the southern California Current system in response to El Niño variability. Prog. Oceanogr. 193, 102544 (2021).
Zeidberg, L. D. & Robison, B. H. Invasive range expansion by the Humboldt squid, Dosidicus gigas, in the eastern North Pacific. Proc. Natl Acad. Sci. USA 104, 12948–12950 (2007).
Szesciorka, A. R. et al. Timing is everything: drivers of interannual variability in blue whale migration. Sci. Rep. 10, 7710 (2020).
Hoving, H.-J. et al. Extreme plasticity in life‐history strategy allows a migratory predator (jumbo squid) to cope with a changing climate. Glob. Change Biol. 19, 2089–2103 (2013).
Boscolo-Galazzo, F. et al. Temperature controls carbon cycling and biological evolution in the ocean twilight zone. Science 371, 1148–1152 (2021).
Deutsch, C., Ferrel, A., Seibel, B. A., Pörtner, H.-O. & Huey, R. B. Climate change tightens a metabolic constraint on marine habitats. Science 348, 1132–1135 (2015).
Seibel, B. A. & Deutsch, C. Oxygen supply capacity in animals evolves to meet maximum demand at the current oxygen partial pressure regardless of size or temperature. J. Exp. Biol. 223, jeb210492 (2020).
Deutsch, C., Penn, J. L. & Seibel, B. A. Diverse hypoxia and thermal tolerances shape biogeography of marine animals. Nature 585, 557–562 (2020).
Childress, J. J. Are there physiological and biochemical adaptations of metabolism in deep-sea animals? Trends Ecol. Evol. 10, 30–36 (1995).
Seibel, B. A. & Drazen, J. C. The rate of metabolism in marine animals: environmental constraints, ecological demands and energetic opportunities. Philos. Trans. R. Soc. B. 362, 2061–2078 (2007).
Seibel, B. A. et al. Oxygen supply capacity breathes new life into the critical oxygen partial pressure (Pcrit). J. Exp. Biol. 224, jeb242210 (2021).
Childress, J. J. & Seibel, B. A. Life at stable low oxygen: adaptations of animals to oceanic oxygen minimum layers. J. Exp. Biol. 201, 1223–1232 (1998).
Garcia, H. E., et al. World Ocean Atlas 2018, Volume 3: Dissolved Oxygen, Apparent Oxygen Utilization, and Oxygen Saturation (NOAA/NESDIS, 2019).
Locarnini, R. A., et. al. World Ocean Atlas 2018, Volume 1: Temperature (NOAA/NESDIS, 2019).
Maas, A. E., Frazar, S., Outram, D., Seibel, B. A. & Wishner, K. F. Fine-scale vertical distribution of macroplankton and micronekton in an eastern tropical North Pacific in association with an oxygen minimum zone. J. Plankton Res. 36, 1557–1575 (2014).
Rosa, R. & Seibel, B. A. Synergistic effect of climate-related variables suggests future physiological impairment in a top oceanic predator. Proc. Natl Acad. Sci. USA 52, 20776–20780 (2008).
Halsey, L. G., Killen, S. S., Clark, T. D. & Norin, T. Exploring key issues of aerobic scope interpretation in ectotherms: absolute versus factorial. Rev. Fish. Biol. Fish. 28, 405–415 (2018).
Peterson, C. C., Nagy, K. A. & Diamond, J. Sustained metabolic scope. Proc. Natl Acad. Sci. USA 87, 2324–2328 (1990).
Seibel, B. A., Luu, B. E., Tessier, S. N., Towanda, T. & Storey, K. B. Metabolic suppression in the pelagic crab, Pleuroncodes planipes, in oxygen minimum zones. Comp. Biochem. Physiol. A 224, 88–97 (2018).
Hadj-Moussa, H., Logan, S. M., Seibel, B. A. & Storey, K. B. Potential role for microRNA in regulating hypoxia-induced metabolic suppression in the jumbo squid? BBA Gene Regul. Mech. 1861, 586–593 (2018).
Torres, J. J. & Childress, J. J. Relationship of oxygen consumption to swimming speed in Euphausia pacifica. Mar. Biol. 74, 79–86 (1983).
Cohen, J. H. & Forward, R. B. Jr. Zooplankton diel vertical migration—a review of proximate control. Oceanogr. Mar. Biol. Annu. Rev. 47, 77–110 (2009).
Gilly, W. F. et al. Locomotion and behavior of Humboldt squid, Dosidicus gigas, in relation to natural hypoxia in the Gulf of California, Mexico. J. Exp. Biol. 215, 3175–3190 (2012).
Jaffe, J. S., Ohman, M. D. & De Robertis, A. Sonar estimates of daytime activity levels of Euphausia pacifica in Saanich inlet. Can. J. Fish. Aquat. Sci. 56, 2000–2010 (1999).
Klevjer, T. A. & Kaartvedt, S. Krill (Meganyctiphanes norvegica) swim faster at night. Limnol. Oceanogr. 56, 765–774 (2011).
Backus, R. H. et al. Ceratoscopelus maderensis: pecuiiar sound-scattering layer identified with this myctophid fish. Science 160, 991–993 (1968).
Barham, E. G. in Proceedings of an International Symposium on Biological Sound Scattering in the Ocean (ed. Farquhar, G. B.) 100–118 (Superintendent of Documents, 1971).
Sanders, N. K. & Childress, J. J. A comparison of the respiratory function of the haemocyanins of vertically migrating and non-migrating pelagic, deep-sea Oplophorid shrimps. J. Exp. Biol. 152, 167–187 (1990).
Seibel, B. A. Critical depth in the jumbo squid, Dosidicus gigas (Ommastrephidae), living in oxygen minimum zones II. Blood-oxygen binding. Deep Sea Res. Part II 95, 139–144 (2013).
Pörtner, H.-O., Bock, C. & Mark, F. C. Oxygen- and capacity-limited thermal tolerance: bridging ecology and physiology. J. Exp. Biol. 220, 2685–2696 (2017).
Laffoley, D. & Baxter, J. M. Ocean Deoxygenation: Everyone’s Problem—Causes, Impacts, Consequences and Solutions (IUCN, 2019).
Birk, M. A. Respirometry: Tools for Conducting and Analyzing Respirometry Experiments. R version 1.4.0 http://cran.r-project.org/package=respirometry (2021).
Huang, B. et al. Improvements of the daily optimum interpolation sea surface temperature (DOISST) Version 2.1. J. Clim. 34, 2923–2939 (2021).
Acknowledgements
This manuscript benefited from thoughtful review by W. Verberk and T. Shaw. A. K. Smith and T. Shaw contributed to collection of the respirometry data. T. Shaw confirmed euphausiid species identifications. K. Wishner contributed to specimen collection and grant management. This project was supported by National Science Foundation grant OCE-1459243 and NOAA grant NA18NOS4780167 to B.A.S. and a National Science Foundation postdoctoral fellowship (DBI-1907197) to M.A.B.
Author information
Authors and Affiliations
Contributions
B.A.S. conceived of the project and wrote the original draft. Both B.A.S. and M.A.B. contributed to all other aspects of the manuscript including methodology, validation, formal analysis, data curation and review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Climate Change thanks Tayler Clarke, Yun-wei Dong and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Dosidicus gigas: Temperature and mass effects on metabolic traits.
a. The temperature dependence, E (eV), of each metric (SMR, MMR, PcSMR and α, was determined from the slope of the linear regression of 1/kBT versus the natural logarithm of each metric (for Dosidicus gigas), where T is absolute temperature (K) and kB is the Boltzmann constant. Note that, to avoid the confusion when using inverse temperature plots, the sign for each temperature coefficient is reversed (that is temperature coefficients are positive when a metric increases with temperature). b. Oxygen-consumption rates recorded at rest (blue, mass-normalized, SMR) and during exercise (MMR, red). The effect of temperature between 10 and 20 °C is similar for MMR and SMR. The effect of temperature is much higher for MMR compared to SMR between 20 and 25 °C. The metabolic rates are normalized to a common body mass (100 g) using the scaling coefficients in panel C. c. SMR and PcSMR as a function of body mass (M) at 10 °C (SMR = 13.03 M−0.12 and PcSMR = 2.44 M−0.03). d. The effect of temperature coefficient on MMR in the Gulf of California (solid lines) and in the California Current System (dashed lines) at different temperatures. The difference between dashed and solid lines at a given temperature (arrows) is due to oxygen limitation in the Gulf of California and the lack of oxygen limitation in the California Current. The measured rates are indicated by black dashed lines. This figure indicates that a temperature coefficient for MMR less than 1.0 eV results in oxygen limitation of maximum metabolic rate whereas a coefficient greater than 1.0 eV results in temperature depression of metabolism at cold temperatures and no effect of low oxygen except at the coldest, deepest daytime depths (~300 m, 10 °C, PO2 < 0.5 kPa).
Extended Data Fig. 2 The precision of derived metrics based on the variability of measured physiological metrics.
A Monte Carlo simulation was conducted for each measured metric (that is SMR, MMR, and PcSMR) of 100,000 draws from a normal distribution with each measured metric’s mean and standard error. For each simulation, FAS = MMR/SMR, alpha = SMR/PcSMR, and Pcmax = MMR/α. FAS is higher at 25 °C (red) than 10 (blue) or 20 °C (green), and Pcmax at 10 and 20 °C is much lower than at 25 °C where it averages near air saturation.
Extended Data Fig. 3 Sensitivity analysis for each measured metabolic metric.
Top row, the modelled factorial aerobic scope (FAS; centre) and the lower (left) and upper (right) confidence band. The lower panels show confidence interval isolines for each metric. In surface waters at low latitudes, for example, the modelled PcSMR used to create Fig. 4c has a 95% CI range of 2.6 kPa (that is ± 1.3 kPa). For most of the metrics, the greatest variability is at higher temperatures where the underlying physiological measurements are higher and more variable and temperature sensitivities (E) are high.
Extended Data Fig. 4 Modelled factorial aerobic scope (FAS) across a latitudinal and depth transect for four euphausiid species.
Top) Yellow band shows the geographic area included in analysis 500 to 2000 km off the western coast of the Americas between 55°S and 55°N. In each subsequent panel pair, the top panel is calculated assuming the temperature coefficient (E) for MMR is 0.3 eV and the bottom panel assumes EMMR = 1.0. The grey regions indicate that PO2 is less than PcSMR and metabolic scope is nil. With the higher temperature coefficient, metabolically suitable habitat (FAS > 3) is present only in the upper 200 m from about 15°S to 30°N, with highest FAS achieved only in the native range. More poleward habitat is constrained due to the high temperature coefficient. With a low-temperature coefficient, the poleward latitudes are metabolically available but underfilled by these tropical migrators.
Extended Data Fig. 5 The interrelationships between metabolic traits provides quantitative projection of aerobic scope.
Pcmax and PcSMR (top); MMR and SMR (middle) and FAS (bottom) as a function of temperature in three different regions using representative oxygen and temperature profiles (inset, right). Yellow shading represents the achievable factorial aerobic scope (lower of FAST or FASPO2). MMRPO2 and FASPO2 (equivalent to the Metabolic Index27; blue lines) are accurate when PO2 is less than Pcmax (O2-limited). MMRT and FAST (orange lines) are accurate when PO2 is greater than Pcmax (not O2-limited). Temperature trends (orange lines) are calculated using measured rates and coefficients for Dosidicus gigas. Note that, in all three regions, FAS is low at low temperature due to the strong effect of temperature. In the California Current (CCS), FAS is driven by temperature at all depths. In the most hypoxic regions of the Eastern Tropical Pacific (ETP), PO2 is consistently lower than Pcmax and MMR and FAS are, thus, oxygen limited at all depths. In the Gulf of California (GoC), oxygen becomes limiting at depth (cold) or at high temperatures where Pcmax is projected to exceed air saturation. No measurements have been made at temperatures beyond 25 °C. Dashed black lines indicate FAS = 1, where MMR = SMR and Pcmax = PcSMR.
Extended Data Fig. 6 Schematic projections of current and future metabolically available habitat in tropical vertical migrators.
The restriction on habitable space imposed by cold (upper left) in temperate waters is relaxed with future warming (upper right). Temperature and oxygen currently impose minimal restrictions on shallow and deep habitat, respectively, at tropical latitudes (lower left). Climate change may exacerbate these constraints to some extent by compressing metabolically available habitat (lower right). Metabolically available habitat refers to temperature and oxygen conditions resulting in FAS > 3. Oxygen limited (black shading, PO2 < Pcmax < 21 kPa); cold limited (blue shading, Pcmax < PO2); heat-limited (red shading, MMR constrained at temperatures > 25 °C by constant PO2 = 21 kPa).
Extended Data Fig. 7 Representative respirometry trials for Tessarabrachion oculatum (Euphausiidae; krill), showing six trials, three at each temperature (10 and 20 °C).
In each trial, the red line, with slope, α (oxygen supply capacity), describes the metabolic rate dependence of PcSMR (PcSMR = MR/α).
Extended Data Fig. 8 Representative respirometry trials for Nyctiphanes simplex (Euphausiidae; krill), showing six trials, three at each temperature (10 and 20 °C).
In each trial, the red line, with slope, α (oxygen supply capacity), describes the metabolic rate dependence of PcSMR (PcSMR = MR/α).
Extended Data Fig. 9 Representative respirometry trials for Nematobrachion flexipes (Euphausiidae; krill), showing six trials, three at each temperature (10 and 20 °C).
In each trial, the red line, with slope, α (oxygen supply capacity), describes the metabolic rate dependence of PcSMR (PcSMR = MR/α).
Extended Data Fig. 10 Representative respirometry trials for Euphausia mucronata (Euphausiidae; krill), showing six trials, three at each temperature (10 and 20 °C).
In each trial, the red line, with slope, α (oxygen supply capacity), describes the metabolic rate dependence of PcSMR (PcSMR = MR/α).
Supplementary information
Supplementary Information
Supplementary Text, Fig. 1, Tables 1 and 2 and References.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Seibel, B.A., Birk, M.A. Unique thermal sensitivity imposes a cold-water energetic barrier for vertical migrators. Nat. Clim. Chang. 12, 1052–1058 (2022). https://doi.org/10.1038/s41558-022-01491-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41558-022-01491-6
This article is cited by
-
Experimental mining plumes and ocean warming trigger stress in a deep pelagic jellyfish
Nature Communications (2023)
-
Historical mass strandings of jumbo squid (Dosidicus gigas) in the Eastern Pacific Ocean: patterns and possible causes
Marine Biology (2023)
-
Oceanic vertical migrators in a warming world
Nature Climate Change (2022)