Abstract
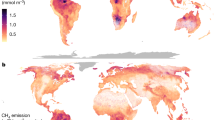
Net emissions of the potent GHG methane from ecosystems represent the balance between microbial methane production (methanogenesis) and oxidation (methanotrophy), each with different sensitivities to temperature. How this balance will be altered by long-term global warming, especially in freshwaters that are major methane sources, remains unknown. Here we show that the experimental warming of artificial ponds over 11 years drives a disproportionate increase in methanogenesis over methanotrophy that increases the warming potential of the gases they emit. The increased methane emissions far exceed temperature-based predictions, driven by shifts in the methanogen community under warming, while the methanotroph community was conserved. Our experimentally induced increase in methane emissions from artificial ponds is, in part, reflected globally as a disproportionate increase in the capacity of naturally warmer ecosystems to emit more methane. Our findings indicate that as Earth warms, natural ecosystems will emit disproportionately more methane in a positive feedback warming loop.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon request. The DNA sequences are in the National Center for Biotechnology Information database, under BioProject ID PRJNA484117. Source data are provided with this paper.
References
Nisbet, E. G., Dlugokencky, E. J. & Bousquet, P. Methane on the rise—again. Science 343, 493–495 (2014).
Balcombe, P., Speirs, J. F., Brandon, N. P. & Hawkes, A. D. Methane emissions: choosing the right climate metric and time horizon. Environ. Sci. Process. Impacts 20, 1323–1339 (2018).
Holgerson, M. A. & Raymond, P. A. Large contribution to inland water CO2 and CH4 emissions from very small ponds. Nat. Geosci. 9, 222–226 (2016).
Saunois, M. et al. The global methane budget 2000–2012. Earth Syst. Sci. Data 8, 697–751 (2016).
Bridgham, S. D., Cadillo-Quiroz, H., Keller, J. K. & Zhuang, Q. Methane emissions from wetlands: biogeochemical, microbial, and modeling perspectives from local to global scales. Glob. Change Biol. 19, 1325–1346 (2013).
Gudasz, C. et al. Temperature-controlled organic carbon mineralization in lake sediments. Nature 466, 478–481 (2010).
Yvon-Durocher, G. et al. Methane fluxes show consistent temperature dependence across microbial to ecosystem scales. Nature 507, 488–491 (2014).
Allen, A. P., Gillooly, J. F. & Brown, J. H. Linking the global carbon cycle to individual metabolism. Funct. Ecol. 19, 202–213 (2005).
Hanson, R. S. & Hanson, T. E. Methanotrophic bacteria. Microbiol. Rev. 60, 439–471 (1996).
Shelley, F., Abdullahi, F., Grey, J. & Trimmer, M. Microbial methane cycling in the bed of a chalk river: oxidation has the potential to match methanogenesis enhanced by warming. Freshw. Biol. 60, 150–160 (2015).
Mohanty, S. R., Bodelier, P. L. E. & Conrad, R. Effect of temperature on composition of the methanotrophic community in rice field and forest soil. FEMS Microbiol. Ecol. 62, 24–31 (2007).
Høj, L., Olsen, R. A. & Torsvik, V. L. Effects of temperature on the diversity and community structure of known methanogenic groups and other archaea in high Arctic peat. ISME J. 2, 37–48 (2008).
Hall, E. K. et al. Understanding how microbiomes influence the systems they inhabit. Nat. Microbiol. 3, 977–982 (2018).
Ho, A., Lüke, C. & Frenzel, P. Recovery of methanotrophs from disturbance: population dynamics, evenness and functioning. ISME J. 5, 750–758 (2011).
Rocca, J. D. et al. Relationships between protein-encoding gene abundance and corresponding process are commonly assumed yet rarely observed. ISME J. 9, 1693–1699 (2015).
Trimmer, M. et al. Riverbed methanotrophy sustained by high carbon conversion efficiency. ISME J. 9, 2304–2314 (2015).
Fey, A. & Conrad, R. Effect of temperature on carbon and electron flow and on the archaeal community in methanogenic rice field soil. Appl. Environ. Microbiol. 66, 4790–4797 (2000).
Ho, A. & Frenzel, P. Heat stress and methane-oxidizing bacteria: effects on activity and population dynamics. Soil Biol. Biochem. 50, 22–25 (2012).
Wilson, R. M. et al. Stability of peatland carbon to rising temperatures. Nat. Commun. 7, 13723 (2016).
Yvon-Durocher, G., Hulatt, C. J., Woodward, G. & Trimmer, M. Long-term warming amplifies shifts in the carbon cycle of experimental ponds. Nat. Clim. Change 7, 209–213 (2017).
Yvon-Durocher, G. et al. Five years of experimental warming increases the biodiversity and productivity of phytoplankton. PLoS Biol. 13, e1002324 (2015).
Davidson, T. A. et al. Synergy between nutrients and warming enhances methane ebullition from experimental lakes. Nat. Clim. Change 8, 156–160 (2018).
McCalley, C. K. et al. Methane dynamics regulated by microbial community response to permafrost thaw. Nature 514, 478–481 (2014).
Conrad, R. Contribution of hydrogen to methane production and control of hydrogen concentrations in methanogenic soils and sediments. FEMS Microbiol. Ecol. 28, 193–202 (1999).
Wilson, R. M. et al. Hydrogenation of organic matter as a terminal electron sink sustains high CO2:CH4 production ratios during anaerobic decomposition. Org. Geochem. 112, 22–32 (2017).
Hodgkins, S. B. et al. Changes in peat chemistry associated with permafrost thaw increase greenhouse gas production. Proc. Natl Acad. Sci. USA 111, 5819–5824 (2014).
Glissmann, K., Chin, K. J., Casper, P. & Conrad, R. Methanogenic pathway and archaeal community structure in the sediment of eutrophic Lake Dagow: effect of temperature. Microb. Ecol. 48, 389–399 (2004).
Inglett, K. S., Inglett, P. W., Reddy, K. R. & Osborne, T. Z. Temperature sensitivity of greenhouse gas production in wetland soils of different vegetation. Biogeochemistry 108, 77–90 (2012).
Conrad, R., Klose, M. & Noll, M. Functional and structural response of the methanogenic microbial community in rice field soil to temperature change. Environ. Microbiol. 11, 1844–1853 (2009).
Metje, M. & Frenzel, P. Methanogenesis and methanogenic pathways in a peat from subarctic permafrost. Environ. Microbiol. 9, 954–964 (2007).
Nozhevnikova, A. N. et al. Influence of temperature and high acetate concentrations on methanogenesis in lake sediment slurries. FEMS Microbiol. Ecol. 62, 336–344 (2007).
Wen, X. et al. Global biogeographic analysis of methanogenic archaea identifies community-shaping environmental factors of natural environments. Front. Microbiol. 8, 1339 (2017).
Conrad, R. et al. Stable carbon isotope discrimination and microbiology of methane formation in tropical anoxic lake sediments. Biogeosciences 8, 795–814 (2011).
Kotsyurbenko, O. R. Trophic interactions in the methanogenic microbial community of low-temperature terrestrial ecosystems. FEMS Microbiol. Ecol. 53, 3–13 (2005).
Yvon-Durocher, G., Montoya, J. M., Woodward, G., Jones, J. I. & Trimmer, M. Warming increases the proportion of primary production emitted as methane from freshwater mesocosms. Glob. Change Biol. 17, 1225–1234 (2011).
Reim, A., Lüke, C., Krause, S., Pratscher, J. & Frenzel, P. One millimetre makes the difference: high-resolution analysis of methane-oxidizing bacteria and their specific activity at the oxic–anoxic interface in a flooded paddy soil. ISME J. 6, 2128–2139 (2012).
Yver Kwok, C. E. et al. Methane emission estimates using chamber and tracer release experiments for a municipal waste water treatment plant. Atmos. Meas. Tech. 8, 2853–2867 (2015).
Sanders, I. A. et al. Emission of methane from chalk streams has potential implications for agricultural practices. Freshw. Biol. 52, 1176–1186 (2007).
Neubacher, E. C., Parker, R. E. & Trimmer, M. Short-term hypoxia alters the balance of the nitrogen cycle in coastal sediments. Limnol. Oceanogr. 56, 651–665 (2011).
R: a language and environment for statistical computing v.3.2.5 (R Core Team, 2014).
Kuznetsova, A., Brockhoff, P. B. & Christensen, R. H. B. {lmerTest} package: tests in linear mixed effects models. J. Stat. Softw. 82, 1–26 (2017).
Lenth, R. emmeans: estimated marginal means, aka least-squares means. R package v.1.4.7 (2019); https://cran.r-project.org/package=emmeans
Nicholls, J. C. & Trimmer, M. Widespread occurrence of the anammox reaction in estuarine sediments. Aquat. Microb. Ecol. 55, 105–113 (2009).
Lever, M. A. & Teske, A. P. Diversity of methane-cycling archaea in hydrothermal sediment investigated by general and group-specific PCR primers. Appl. Environ. Microbiol. 81, 1426–1441 (2015).
Horz, H. P., Rich, V., Avrahami, S. & Bohannan, B. J. M. Methane-oxidizing bacteria in a California upland grassland soil: diversity and response to simulated global change. Appl. Environ. Microbiol. 71, 2642–2652 (2005).
Caporaso, J. G. et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 7, 335–336 (2010).
King, T., Butcher, S. & Zalewski, L. Apocrita—High Performance Computing Cluster for Queen Mary University of London (Queen Mary University of London, 2017); https://doi.org/10.5281/ZENODO.438045
Callahan, B. J. et al. DADA2: high-resolution sample inference from Illumina amplicon data. Nat. Methods 13, 581–583 (2016).
Pester, M., Friedrich, M. W., Schink, B. & Brune, A. pmoA-based analysis of methanotrophs in a littoral lake sediment reveals a diverse and stable community in a dynamic environment. Appl. Environ. Microbiol. 70, 3138–3142 (2004).
Oakley, B. B., Carbonero, F., Dowd, S. E., Hawkins, R. J. & Purdy, K. J. Contrasting patterns of niche partitioning between two anaerobic terminal oxidizers of organic matter. ISME J. 6, 905–914 (2012).
Wilkins, D., Lu, X. Y., Shen, Z., Chen, J. & Lee, P. K. H. Pyrosequencing of mcrA and archaeal 16s rRNA genes reveals diversity and substrate preferences of methanogen communities in anaerobic digesters. Appl. Environ. Microbiol. 81, 604–613 (2015).
Yang, S., Wen, X. & Liebner, S. pmoA Gene Reference Database (Fasta-Formatted Sequences and Taxonomy) (GFZ Data Services, 2016).
McMurdie, P. J. & Holmes, S. phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 8, e61217 (2013).
Anderson, M. J. in Wiley StatsRef: Statistics Reference Online 1–15 (Wiley, 2017).
Oksanen, J. et al. vegan: community ecology package. R package v.2.5-6 (2018); https://cran.r-project.org/package=vegan
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Acknowledgements
This study was supported by Queen Mary University of London and the UK Natural Environment Research Council (grant nos NE/M02086X/1 and NE/M020886/1). We thank I. Sanders and F. Shelley for technical and fieldwork assistance, J. Pretty for mesocosm pond maintenance, M. Rouen for designing and installing the Campbell control and data-logging system, H. Prentice for collecting sediments and for DNA extraction, C. Economou and M. Struebig for help with the molecular work, and P. K. H. Lee for providing the mcrA database and related documents for the bioinformatics analysis. We thank the principal investigators of the methane flux data products, including W. Quinton, O. Sonnentag, G. Wohlfahrt, S. Gogo, T. Schuur, K. Krauss, A. Desai, G. Bohrer, R. Vargas, D. Baldocchi, J. Chen, H. Chu, H. Iwata, M. Ueyama and Y. Harazono. We also thank the funding agencies that supported their flux measurements.
Author information
Authors and Affiliations
Contributions
M.T., Y.Z. and K.J.P conceived the study. Y.Z. conducted the vast majority of the experiments and analysed the data. Y.Z., M.T., K.J.P., G.Y.-D. and A.J.D. discussed the data. Y.Z., M.T. and K.J.P. wrote the manuscript, and all authors contributed to revisions. Y.Z. and S.F.H. set up the chamber system. Y.Z., O.E. and L.S. performed the molecular analyses.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Schematic of experimental pond set-up and dynamic chamber measurements.
Twenty artificial ponds, with 10 warmed (red) by 4 °C above 10 ambient (blue) ponds, were paired in a randomized block design (a) and controlled via two temperature sensors (T1, T2), a thermocouple (T-stat) and a solid-state relay (SSR) (b). Dynamic LI-COR chambers, floating on lifebuoys, were installed on 7 each of the warmed and ambient ponds (c). Each floating chamber was connected to one of the inlet ports on the MIU and the MIU outlet port was connected to the gas inlet port of Ultra-Portable Greenhouse Gas Analyzer (LGR) (d). A dynamic chamber is sequentially triggered to close by a customised Campbell control unit (CCU) for 30 minutes for gas measurements while the other chambers remain open. When a chamber is triggered to close, the MIU switches simultaneously to the inlet connected to the closing chamber to direct its gas flow to the LGR. See Methods and Extended Data Fig. 2 for further details on methane emissions.
Extended Data Fig. 2 Consistent seasonal patterns in daily methane emissions under warming but with ongoing divergence over 10 years (200735, 201320 and 2017 (this study)).
The seasonal patterns in all 3 years are very similar, despite the use of different techniques but the frequent measurements (three times daily) using dynamic chambers in 2017 captured far more details in emissions compared to 2007 and 2013 when static chambers were used to measure methane emission on 7 and 12 occasions over each year, respectively. Note the natural log scale for methane emissions.
Extended Data Fig. 3 Methane emissions at night and during the day.
Methane emissions during the day (a) and at night (b) follow similar seasonal patterns; yet methane emissions at night are significantly greater than during the day (c).
Supplementary information
Supplementary Information
Supplementary Figs. 1–6, Tables 1–10 and discussion.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Rights and permissions
About this article
Cite this article
Zhu, Y., Purdy, K.J., Eyice, Ö. et al. Disproportionate increase in freshwater methane emissions induced by experimental warming. Nat. Clim. Chang. 10, 685–690 (2020). https://doi.org/10.1038/s41558-020-0824-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41558-020-0824-y
This article is cited by
-
High methane ebullition throughout one year in a regulated central European stream
Scientific Reports (2024)
-
Type I methanotrophs dominated methane oxidation and assimilation in rice paddy fields by the consequence of niche differentiation
Biology and Fertility of Soils (2024)
-
Global methane emissions from rivers and streams
Nature (2023)
-
Thermal acclimation of methanotrophs from the genus Methylobacter
The ISME Journal (2023)
-
Direct biological fixation provides a freshwater sink for N2O
Nature Communications (2023)