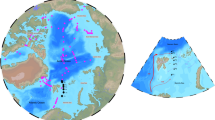
Abstract
Increasing atmospheric CO2 levels are largely absorbed by the ocean, decreasing surface water pH1. In combination with increasing ocean temperatures, these changes have been identified as a major sustainability threat to future marine life2. Interactions between marine organisms are known to depend on biomolecules, although the influence of oceanic pH on their bioavailability and functionality remains unexplored. Here we show that global change substantially impacts two ecological keystone molecules3 in the ocean, the paralytic neurotoxins saxitoxin and tetrodotoxin. Increasing temperatures and declining pH increase the abundance of their toxic forms in the water. Our geospatial global model predicts where this increased toxicity could intensify the devastating impact of harmful algal blooms, for example through an increased incidence of paralytic shellfish poisoning. Calculations of future saxitoxin toxicity levels in Alaskan clams, Saxidomus gigantea, show critical exceedance of limits safe for consumption. Our findings for saxitoxin and tetrodotoxin exemplify potential consequences of changing pH and temperature on chemicals dissolved in the sea. This reveals major implications not only for ecotoxicology, but also for chemical signals that mediate species interactions such as foraging, reproduction or predation in the ocean, with unexplored consequences for ecosystem stability and ecosystem services.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
Source data for curves in Fig. 1 calculated based on the given references and coordinates of the molecular structures are available from the corresponding author upon request. The data used to generate the dataset for Fig. 2 are available from the Harmful Algal Information System metadatabase (HAEDAT, http://haedat.iode.org), the NOAA COPEPOD database (https://www.st.nmfs.noaa.gov/copepod), the Global marine environment dataset (GMED, http://gmed.auckland.ac.nz) and the IPCC (WCRP CMPI3) multi-model database (https://cmip.llnl.gov). Data used to generate Fig. 3 can be accessed via the website of the Qagan Tayagungin Tribe (https://www.qttribe.org>Environment>PSP Program). The extracted, collated data supporting the findings in our study are deposited in the PANGAEA archive and available at https://doi.org/10.1594/PANGAEA.904260.
Code availability
The code used to calculate the proportions of different protonation states is available from the corresponding author on request .
References
IPCC Climate Change 2014: Synthesis Report (eds Core Writing Team, Pachauri, R. K. & Meyer L. A.) (IPCC, 2014).
DeWeerdt, S. Sea change. Nature 550, S54–S58 (2017).
Ferrer, R. P. & Zimmer, R. K. Molecules of keystone significance: crucial agents in ecology and resource management. Bioscience 63, 428–438 (2013).
Caldeira, K. & Wickett, M. E. Oceanography: anthropogenic carbon and ocean pH. Nature 425, 365 (2003).
Baumann, H. & Smith, E. M. Quantifying metabolically driven pH and oxygen fluctuations in US nearshore habitats at diel to interannual time scales. Estuaries Coasts 41, 1102–1117 (2018).
Fabry, V. J., Seibel, B. A., Feely, R. A. & Orr, J. C. Impacts of ocean acidification on marine fauna and ecosystem processes. ICES J. Mar. Sci. 65, 414–432 (2008).
Wittmann, A. C. & Pörtner, H.-O. Sensitivities of extant animal taxa to ocean acidification. Nat. Clim. Change 3, 995–1001 (2013).
Clements, J. C. & Hunt, H. L. Marine animal behaviour in a high CO2 ocean. Mar. Ecol. Prog. Ser. 536, 259–279 (2015).
Roggatz, C. C., Lorch, M., Hardege, J. D. & Benoit, D. M. Ocean acidification affects marine chemical communication by changing structure and function of peptide signalling molecules. Glob. Change Biol. 22, 3914–3926 (2016).
Porteus, C. S. et al. Near-future CO2 levels impair the olfactory system of a marine fish. Nat. Clim. Change 8, 737–743 (2018).
Hay, M. E. Marine chemical ecology: chemical signals and cues structure marine populations, communities, and ecosystems. Ann. Rev. Mar. Sci. 1, 193–212 (2009).
Bane, V., Lehane, M., Dikshit, M., O’Riordan, A. & Furey, A. Tetrodotoxin: chemistry, toxicity, source, distribution and detection. Toxins 6, 693–755 (2014).
Cusick, K. D. & Sayler, G. S. An overview on the marine neurotoxin, saxitoxin: genetics, molecular targets, methods of detection and ecological functions. Mar. Drugs 11, 991–1018 (2013).
Williams, B. L. Behavioral and chemical ecology of marine organisms with respect to tetrodotoxin. Mar. Drugs 8, 381–398 (2010).
Wyatt, T. & Jenkinson, I. R. Notes on Alexandrium population dynamics. J. Plankton Res. 19, 551–575 (1997).
Lefebvre, K. A. et al. Characterization of intracellular and extracellular saxitoxin levels in both field and cultured Alexandrium spp. samples from Sequim Bay, Washington. Mar. Drugs 6, 103–116 (2008).
Gobler, C. J. et al. Ocean warming since 1982 has expanded the niche of toxic algal blooms in the North Atlantic and North Pacific oceans. Proc. Natl Acad. Sci. USA 114, 4975–4980 (2017).
Riebesell, U. et al. Toxic algal bloom induced by ocean acidification disrupts the pelagic food web. Nat. Clim. Change 8, 1082–1086 (2018).
Li, A., Chen, H., Qiu, J., Lin, H. & Gu, H. Determination of multiple toxins in whelk and clam samples collected from the Chukchi and Bering seas. Toxicon 109, 84–93 (2016).
Starr, M. et al. Multispecies mass mortality of marine fauna linked to a toxic dinoflagellate bloom. PLoS ONE 12, e0176299 (2017).
Wang, D.-Z. Neurotoxins from marine dinoflagellates: a brief review. Mar. Drugs 6, 349–371 (2008).
Woodward, R. B. The structure of tetrodotoxin. J. Macromol. Sci. Pure Appl. Chem. 9, 49–74 (1964).
Rogers, R. S. & Rapoport, H. The pK as of saxitoxin. J. Am. Chem. Soc. 102, 7335–7339 (1980).
Hegyi, B. et al. Tetrodotoxin blockade on canine cardiac L-type Ca2+ channels depends on pH and redox potential. Mar. Drugs 11, 2140–2153 (2013).
Yotsu-Yamashita, M., Sugimoto, A., Takai, A. & Yasumoto, T. Effects of specific modifications of several hydroxyls of tetrodotoxin on its affinity to rat brain membrane. J. Pharmacol. Exp. Ther. 289, 1688–1696 (1999).
Roggatz, C. C., Lorch, M. & Benoit, D. M. Influence of solvent representation on nuclear shielding calculations of protonation states of small biological molecules. J. Chem. Theory Comput. 14, 2684–2695 (2018).
Colquhoun, D., Henderson, R. & Ritchie, J. M. The binding of labelled tetrodotoxin to non-myelinated nerve fibres. J. Physiol. 227, 95–126 (1972).
Ulbricht, W. & Wagner, H. H. The influence of pH on the rate of tetrodotoxin action on myelinated nerve fibres. J. Physiol. 252, 185–202 (1975).
Natsuike, M. et al. Possible spreading of toxic Alexandrium tamarense blooms on the Chukchi Sea shelf with the inflow of Pacific summer water due to climatic warming. Harmful Algae 61, 80–86 (2017).
Blackburn, S. I., Hallegraeff, G. M. & Bolch, C. J. Vegetative reproduction and sexual life cycle of the toxic dinoflagellate Gymnodinium catenatum from Tasmania, Australia. J. Phycol. 25, 577–590 (1989).
Hallegraeff, G. M. Ocean climate change, phytoplankton community responses, and harmful algal blooms: a formidable predictive challenge. J. Phycol. 46, 220–235 (2010).
Hallegraeff, G. M. A review of harmful algal blooms and their apparent global increase. Phycologia 32, 79–99 (1993).
Tatters, A. O., Flewelling, L. J., Fu, F., Granholm, A. A. & Hutchins, D. A. High CO2 promotes the production of paralytic shellfish poisoning toxins by Alexandrium catenella from Southern California waters. Harmful Algae 30, 37–43 (2013).
Cembella, A. in Physiological Ecology of Harmful Algal Blooms (eds. Anderson, D. M. et al.) 381–404 (NATO ASI Series G 41, Springer, 1998).
Lefebvre, K. A. et al. Prevalence of algal toxins in Alaskan marine mammals foraging in a changing Arctic and Subarctic environment. Harmful Algae 55, 13–24 (2016).
Zonneveld, K. A. F. et al. Atlas of modern dinoflagellate cyst distribution based on 2405 data points. Rev. Palaeobot. Palynol. 191, 1–197 (2013).
Bezençon, J. et al. pK a determination by 1H NMR spectroscopy—an old methodology revisited. J. Pharm. Biomed. Anal. 93, 147–155 (2014).
Goto, T., Kishi, Y., Takahashi, S. & Hirata, Y. Tetrodotoxin. Tetrahedron 21, 2059–2088 (1965).
Shimizu, Y. et al. Structure of neosaxitoxin. J. Am. Chem. Soc. 100, 6791–6793 (1978).
Po, H. N. & Senozan, N. M. The Henderson–Hasselbalch equation: its history and limitations. J. Chem. Educ. 78, 1499 (2001).
Reijenga, J. C., Gagliardi, L. G. & Kenndler, E. Temperature dependence of acidity constants, a tool to affect separation selectivity in capillary electrophoresis. J. Chromatogr. A 1155, 142–145 (2007).
Global Climate Report—Annual 2018 State of the Climate (National Centers for Environmental Information, 2019).
Kim, S. et al. PubChem substance and compound databases. Nucleic Acids Res. 44, D1202–D1213 (2016).
Mosher, H. S. The chemistry of tetrodotoxin. Ann. N. Y. Acad. Sci. 479, 32–43 (1986).
Adamo, C. & Barone, V. Toward reliable density functional methods without adjustable parameters: the PBE0 model. J. Chem. Phys. 110, 6158–6170 (1999).
Jensen, F. Polarization consistent basis sets: principles. J. Chem. Phys. 115, 9113–9125 (2001).
Jensen, F. Polarization consistent basis sets. II. Estimating the Kohn–Sham basis set limit. J. Chem. Phys. 116, 7372–7379 (2002).
Jensen, F. Polarization consistent basis sets. III. The importance of diffuse functions. J. Chem. Phys. 117, 9234–9240 (2002).
Klamt, A. Conductor-like screening model for real solvents: a new approach to the quantitative calculation of solvation phenomena. J. Phys. Chem. 99, 2224–2235 (1995).
Neese, F. The ORCA program system. WIREs Comput. Mol. Sci. 2, 73–78 (2012).
Neese, F., Wennmohs, F., Hansen, A. & Becker, U. Efficient, approximate and parallel Hartree–Fock and hybrid DFT calculations. A ‘chain-of-spheres’ algorithm for the Hartree–Fock exchange. Chem. Phys. 356, 98–109 (2009).
Weigend, F. & Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 7, 3297–3305 (2005).
Grimme, S., Antony, J., Ehrlich, S. & Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H–Pu. J. Chem. Phys. 132, 154104 (2010).
Bode, B. M. & Gordon, M. S. MacMolPlt: a graphical user interface for GAMESS. J. Mol. Graph. Model. 16, 164 (1998).
Basher, Z., Costello, M. J. & Bowden, D. A. Global Marine Environment Dataset (GMED) (Univ. Auckland, 2015).
Boyer, T. P. et al. World Ocean Database 2013 (National Centers for Environmental Information, 2013); https://www.nodc.noaa.gov/OC5/WOD13/
Tyberghein, L. et al. Bio-ORACLE: a global environmental dataset for marine species distribution modelling. Glob. Ecol. Biogeogr. 21, 272–281 (2012).
Gattuso, J.-P. et al. Contrasting futures for ocean and society from different anthropogenic CO2 emissions scenarios. Science 349, aac4722 (2015).
World Ocean Basemap (ArcGIS REST Services Directory, ESRI, accessed 28 May 2018); http://services.arcgisonline.com/arcgis/rest/services/Ocean/World_Ocean_Base/MapServer
PSP Program (Quagan Tayagungin Tribe, accessed 14 August 2018); https://go.nature.com/2kXEn85
Ringwood, A. H. & Keppler, C. J. Water quality variation and clam growth: Is pH really a non-issue in estuaries? Estuaries 25, 901–907 (2002).
Booth, C. E., McDonald, D. G. & Walsh, P. J. Acid-base balance in the sea mussel, Mytilus edulis. I. Effects of hypoxia and air-exposure on hemolymph acid-base status. Mar. Biol. Lett. 5, 347–358 (1984).
Dwyer, J. J. III & Burnett, L. E. Acid-base status of the oyster Crassostrea virginica in response to air exposure and to infections by Perkinsus marinus. Biol. Bull. 190, 139–147 (1996).
Fish and Fishery Products Hazards and Controls Guidance (Department of Health and Human Services, Public Health Service, Food and Drug Administration, Center for Food Safety and Applied Nutrition, Office for Food Safety, 2011).
Acknowledgements
We acknowledge the Viper High Performance Computing facility of the University of Hull and its support team. C.C.R. was funded through D. Parsons’ project grant (no. ERC-2016-COG GEOSTICK). We thank the Quagan Tayagungin Tribe for access to the clam toxicity data at the PSP Program website. We acknowledge the World Climate Research Programme’s Working Group on Coupled Modelling and the climate modelling groups for producing and making available their model output. For CMIP the US Department of Energy’s Program for Climate Model Diagnosis and Inter-comparison provides coordinating support and led the development of software infrastructure in partnership with the Global Organization for Earth System Science Portals. We thank D. Parsons, Energy and Environment Institute, University of Hull, and H. Bartels-Hardege, Biological and Marine Sciences, University of Hull, for valuable suggestions and discussions.
Author information
Authors and Affiliations
Contributions
C.C.R. and J.D.H. designed the study. K.C.W.V. and A.C.A. performed the analysis of the datasets for geospatial models and C.C.R. and D.M.B. calculated the molecular models. Access to toxin mussel data was provided by A.D. and B.W. C.C.R, J.D.H. and K.C.W.V. shared responsibility for, and N.F. contributed to, the writing of the manuscript. All authors contributed to the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Climate Change thanks K. Cusick and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary methods, Figs. 1–3, Table 1 and references.
Rights and permissions
About this article
Cite this article
Roggatz, C.C., Fletcher, N., Benoit, D.M. et al. Saxitoxin and tetrodotoxin bioavailability increases in future oceans. Nat. Clim. Chang. 9, 840–844 (2019). https://doi.org/10.1038/s41558-019-0589-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41558-019-0589-3
This article is cited by
-
Algal blooms in the ocean: hot spots for chemically mediated microbial interactions
Nature Reviews Microbiology (2024)
-
Microbial Metabolites Beneficial to Plant Hosts Across Ecosystems
Microbial Ecology (2023)
-
The role of changing pH on olfactory success of predator–prey interactions in green shore crabs, Carcinus maenas
Aquatic Ecology (2022)
-
Modelling Antifouling compounds of Macroalgal Holobionts in Current and Future pH Conditions
Journal of Chemical Ecology (2022)
-
Can small-bodied Daphnia control Raphidiopsis raciborskii in eutrophic tropical lakes? A mesocosm experiment
Environmental Science and Pollution Research (2020)