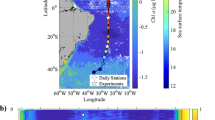
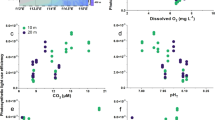
Abstract
Climate change-mediated alteration of Southern Ocean primary productivity is projected to have biogeochemical ramifications regionally, and globally due to altered northward nutrient supply1,2. Laboratory manipulation studies that investigated the influence of the main drivers (CO2, light, nutrients, temperature and iron) on Southern Ocean diatoms revealed that temperature and iron exert major controls on growth under year 2100 conditions3,4. However, detailed physiological studies, targeting temperature and iron, are required to improve our mechanistic understanding of future diatom responses. Here, I show that thermal performance curves of bloom-forming polar species are more diverse than previously shown5, with the optimum temperature for growth (Topt) ranging from 5–16 °C (the annual temperature range is −1–8 °C). Furthermore, iron deficiency probably decreases polar diatom Topt and Tmax (the upper bound for growth), as recently revealed for macronutrients and temperate phytoplankton6. Together, this diversity of thermal performance curves and the physiological interplay between iron and temperature may alter the diatom community composition. Topt will be exceeded during 2100 summer low iron/warmer conditions, tipping some species close or beyond Tmax, but giving others a distinct physiological advantage. Future polar conditions will enhance primary productivity2,3,4, but will also probably cause floristic shifts, such that the biogeochemical roles and elemental stoichiometry of dominant diatom species will alter the polar biogeochemistry and northwards nutrient supply.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
Data on the species and strain identification, collection and maintenance are detailed in Supplementary Table 1. Following publication, these data will be made available (that is, open access) through the IMAS and UTAS (see http://www.imas.utas.edu.au/data). All data collected by IMAS researchers are archived, curated and managed by IMAS, and often supported by related organizations such as the Integrated Marine Observing System, Australian Ocean Data Network and Tasmanian Partnership for Advanced Computing. Our guiding framework is that all data that are not commercial-in-confidence or restricted by legislation should be shared with researchers for analysis and interpretation. IMAS also operates facilities and hosts datasets of national and global interest and for the benefit of the community.
References
Sarmiento, J. L., Gruber, N., Brzezinski, M. A. & Dunne, J. P. High-latitude controls of thermocline nutrients and low latitude biological productivity. Nature 427, 56–60 (2004).
Moore, J. K. et al. Sustained climate warming drives declining marine biological productivity. Science 359, 1139–1143 (2018).
Boyd, P. W. et al. Physiological responses of a Southern Ocean diatom to complex future ocean conditions.Nat. Clim. Change 6, 207–213 (2016).
Xu, K., Fu, F. X. & Hutchins, D. A. Comparative responses of two dominant Antarctic phytoplankton taxa to interactions between ocean acidification, warming, irradiance, and iron availability. Limnol. Oceanogr. 59, 1919–1931 (2014).
Coello-Camba, A. & Agustí, S. Thermal thresholds of phytoplankton growth in polar waters and their consequences for a warming polar ocean. Front. Mar. Sci. 4, 168 (2017).
Thomas, M. K. et al. Temperature–nutrient interactions exacerbate sensitivity to warming in phytoplankton. Glob. Change Biol. 23, 3269–3280 (2017).
Laufkötter, C. & Gruber, N. Will marine productivity wane? Science 359, 1103–1104 (2018).
Leung, S., Cabré, A. & Marinov, I. A latitudinally banded phytoplankton response to 21st century climate change in the Southern Ocean across the CMIP5 model suite. Biogeosciences 12, 5715–5734 (2015).
Behrenfeld, M. J. et al. Annual boom–bust cycles of polar phytoplankton biomass revealed by space-based lidar. Nat. Geosci. 10, 118–122 (2017).
Henson, S. A. et al. Detection of anthropogenic climate change in satellite records of ocean chlorophyll and productivity. Biogeosciences 7, 621–640 (2010).
Assmy, P. et al. Thick-shelled, grazer-protected diatoms decouple ocean carbon and silicon cycles in the iron-limited Antarctic Circumpolar Current. Proc. Natl Acad. Sci. USA 110, 20633–20638 (2013).
Tagliabue, A. et al. Surface-water iron supplies in the Southern Ocean sustained by deep winter mixing. Nat. Geosci. 7, 314–320 (2014).
Murphy, E. J. et al. Comparison of the structure and function of Southern Ocean regional ecosystems: the Antarctic Peninsula and South Georgia. J. Mar. Syst. 109, 22–42 (2013).
Arrigo, K. R. in Sea Ice (ed. Thomas, D. N.) Ch. 14 (Wiley, New York, 2017).
Hutchins, D. A. & Boyd, P. W. Marine phytoplankton and the changing ocean iron cycle. Nat. Clim. Change 6, 1072–1079 (2017).
Mock, T. et al. Evolutionary genomics of the cold-adapted diatom Fragilariopsis cylindrus. Nature 541, 536–540 (2017).
Strzepek, R. F. et al. Iron–light interactions differ in Southern Ocean phytoplankton. Limnol. Oceanogr. 57, 1182–1200 (2012).
Hunt, G. L. Jr et al. Advection in polar and sub-polar environments: impacts on high latitude marine ecosystems. Prog. Oceanogr. 149, 40–81 (2016).
Malviya, S. et al. Insights into global diatom distribution and diversity in the world’s ocean. Proc. Natl Acad. Sci. USA 113, E1516–E1525 (2016).
Fraser, C. I. et al. Antarctica’s ecological isolation will be broken by storm-driven dispersal and warming. Nat. Clim. Change 8, 704–708 (2018).
Boyd, P. W., Lennartz, S. T., Glover, D. M. & Doney, S. C. Biological ramifications of climate-change-mediated oceanic multi-stressors.Nat. Clim. Change 5, 71–79 (2015).
Boyd, P. W., Arrigo, K. R., Strzepek, R. & van Dijken, G. L. Mapping iron demand provides insights into Southern Ocean supply mechanisms. J. Geophys. Res. 117, C06009 (2012).
Kudo, I., Miyamoto, M., Noiri, Y. & Maita, Y. Combined effects of temperature and iron on the growth and physiology of the marine diatom Phaeodactylum tricornutum (Bacillariophyceae). J. Phycol. 36, 1096–1102 (2000).
Marañón, E., Lorenzo, M. P., Cermeño, P. & Mouriño-Carballido, B. Nutrient limitation suppresses the temperature dependence of phytoplankton metabolic rates.ISME. J. 12, 1836–1845 (2018).
Zhang, Y. et al. Between- and within-population variations in thermal reaction norms of the coccolithophore Emiliania huxleyi. Limnol. Oceanogr. 59, 1570–1580 (2014).
Esper, O. & Gersonde, R. Quaternary surface water temperature estimations: new diatom transfer functions for the Southern Ocean. Palaeogeogr. Palaeoclimatol. Palaeoecol. 414, 1–19 (2014).
Thomas, M. K., Kremer, C. T., Klausmeier, C. A. & Litchman, E. A global pattern of thermal adaptation in marine phytoplankton. Science 338, 1085–1088 (2012).
Sunda, W. G. & Huntsman, S. A. Interrelated influence of iron, light and cell size on marine phytoplankton growth. Nature 390, 389–392 (2005).
Dutkiewicz, S., Scott, J. R. & Follows, M. J. Winners and losers: ecological and biogeochemical changes in a warming ocean. Glob. Biogeochem. Cycles 27, 463–477 (2013).
Quéguiner, B. Iron fertilization and the structure of planktonic communities in high nutrient regions of the Southern Ocean. Deep Sea Res. Pt II 90, 43–54 (2013).
Elzhov, T. V., Mullen, K. M., Spiess, A.-N. & Bolker, B. minpack.lm: R Interface to the Levenberg–Marquardt Nonlinear Least-Squares Algorithm Found in MINPACK, Plus Support for Bounds (CRAN Repository, 2016); https://cran.r-project.org/web/packages/minpack.lm/minpack.lm.pdf
R Development Core Team R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2017).
Ratkowsky, D. A., Lowry, R. K., McMeekin, T. A., Stokes, A. N. & Chandler, R. E. Model for bacterial culture growth rate throughout the entire biokinetic temperature range. J. Bacteriol. 154, 1222–1226 (1983).
Acknowledgements
I am grateful to K. Karsh (CSIRO, Australia) for conducting the phytoplankton laboratory culture assays and maintaining the cultures, R. Corkery (TIA, UTAS, Australia) for curve fitting the TPCs, the laboratory of A. McMinn (IMAS, UTAS, Australia) for isolating the polar species, I. Lima (WHOI, USA) and S. Doney (VIMS, USA) for providing the temperature range from NCAR Community Earth System Model 2, J. Llort and P. Strutton (IMAS) for providing the time-series of temperature and chlorophyll a from the profiling bio-floats, and M. Ellwood (ANU, Australia) for sharing unpublished data on C. neglectus. This work was primarily funded by the Antarctic Climate and Ecosystems Cooperative Research Centre, University of Tasmania, Hobart, Australia, and by the Australian Research Council through a Laureate awarded to PWB (FL160100131).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figures 1–4, Supplementary References, Supplementary Table 1, Supplementary Materials
Rights and permissions
About this article
Cite this article
Boyd, P.W. Physiology and iron modulate diverse responses of diatoms to a warming Southern Ocean. Nature Clim Change 9, 148–152 (2019). https://doi.org/10.1038/s41558-018-0389-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41558-018-0389-1
This article is cited by
-
Twenty-six years of phytoplankton pigments reveal a circumpolar Class Divide around the Southern Ocean
Communications Earth & Environment (2024)
-
Effect of dissolved iron (II) and temperature on growth of the Southern Ocean phytoplankton species Fragilariopsis cylindrus and Phaeocystis antarctica
Polar Biology (2023)
-
Marine phytoplankton functional types exhibit diverse responses to thermal change
Nature Communications (2021)
-
Acidification diminishes diatom silica production in the Southern Ocean
Nature Climate Change (2019)