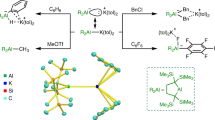
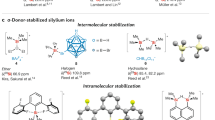
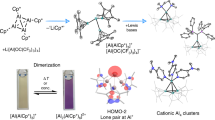
Abstract
Molecular species containing multiple bonds to aluminium have long been challenging synthetic targets. Despite recent landmark discoveries in this area, heterodinuclear Al–E multiple bonds (where E is a group-14 element) have remained rare and limited to highly polarized π-interactions (Al=E ↔ +Al–E–). Here we report the isolation of three alumanyl silanide anions that feature an Al–Si core stabilized by bulky substituents and a Si–Na interaction. Single-crystal X-ray diffraction studies, spectroscopic analysis and density functional theory calculations show that the Al–Si interaction possesses partial double bond character. Preliminary reactivity studies support this description of the compounds through two resonance structures: one that displays a predominant nucleophilic character of the sodium-coordinated silicon centre in the Al–Si core, as shown by silanide-like reactivity towards halosilane electrophiles and the CH-insertion of phenylacetylene. Moreover, we report an alumanyl silanide with a sequestered sodium cation. Cleavage of the Si–Na bond by [2.2.2]cryptand increases the double bond character of the Al–Si core to produce an anion with high aluminata-silene (–Al=Si) character.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data generated or analysed during this study are included in this published Article and its Supplementary Information files. The structures of compounds 2·IMes, 3·NMe3, 4·NMe3, 5·Na·(15-c-5), 5·Na·IiPr, Na·crypt[5], Na·(DME)3[6] and 7 were determined by single-crystal XRD. Crystallographic data for the structures reported in this Article have been deposited at the Cambridge Crystallographic Data Centre, under deposition numbers CCDC 2170414 (2·IMes), 2170415 (3·NMe3), 2170416 (4·NMe3), 2170417 (5·Na·(15-c-5)), 2170418 (5·Na·IiPr), 2170419 (Na·crypt[5]), 2170420 (Na·(DME)3[6]) and 2170421 (7). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/. The Cartesian coordinates of all optimized and calculated structures as well as electronic energies are summarized in the supplementary document ‘calculated structures.txt’. The files comprise all necessary data for reproducing the values. All non-default parameters for the computational studies are given in the Supplementary Information together with the corresponding references of the methods used. Further details are provided in the Supplementary Information.
References
Bag, P., Weetman, C. & Inoue, S. Experimental realisation of elusive multiple-bonded aluminium compounds: a new horizon in aluminium chemistry. Angew. Chem. Int. Ed. 57, 14394–14413 (2018).
Shan, C., Yao, S. & Driess, M. Where silylene-silicon centres matter in the activation of small molecules. Chem. Soc. Rev. 49, 6733–6754 (2020).
Chu, T. & Nikonov, G. I. Oxidative addition and reductive elimination at main-group element centers. Chem. Rev. 118, 3608–3680 (2018).
Power, P. P. Main-group elements as transition metals. Nature 463, 171–177 (2010).
Weetman, C. & Inoue, S. The road travelled: after main‐group elements as transition metals. ChemCatChem 10, 4213–4228 (2018).
West, R., Fink, M. J. & Michl, J. Tetramesityldisilene, a stable compound containing a silicon-silicon double bond. Science 214, 1343–1344 (1981).
Kira, M. Bonding and structure of disilenes and related unsaturated group-14 element compounds. Proc. Jpn Acad. B 88, 167–191 (2012).
Matsuo, T. & Hayakawa, N. π-Electron systems containing Si=Si double bonds. Sci. Technol. Adv. Mater. 19, 108–129 (2018).
Rammo, A. & Scheschkewitz, D. Functional disilenes in synthesis. Chem. Eur. J. 24, 6866–6885 (2018).
Bag, P., Porzelt, A., Altmann, P. J. & Inoue, S. A stable neutral compound with an aluminum-aluminum double bond. J. Am. Chem. Soc. 139, 14384–14387 (2017).
Falconer, R. L., Byrne, K. M., Nichol, G. S., Krämer, T. & Cowley, M. J. Reversible dissociation of a dialumene*. Angew. Chem. Int. Ed. 60, 24702–24708 (2021).
Weetman, C., Porzelt, A., Bag, P., Hanusch, F. & Inoue, S. Dialumenes - aryl vs. silyl stabilisation for small molecule activation and catalysis. Chem. Sci. 11, 4817–4827 (2020).
Allred, A. L. Electronegativity values from thermochemical data. J. Inorg. Nucl. Chem. 17, 215–221 (1961).
Palagyi, Z., Grev, R. S. & Schaefer, H. F. Striking similarities between elementary silicon and aluminum compounds: monobridged, dibridged, trans-bent, and vinylidene isomers of aluminum hydride (Al2H2). J. Am. Chem. Soc. 115, 1936–1943 (1993).
Kysliak, O., Görls, H. & Kretschmer, R. Cooperative bond activation by a bimetallic main-group complex. J. Am. Chem. Soc. 143, 142–148 (2021).
Gil-Negrete, J. M. & Hevia, E. Main group bimetallic partnerships for cooperative catalysis. Chem. Sci. 12, 1982–1992 (2020).
Uhl, W., Vester, A., Kaim, W. & Poppe, J. Dialan-Radikalanionen [R2Al-AlR2]−. J. Organomet. Chem. 454, 9–13 (1993).
Pluta, C., Pörschke, K.-R., Krüger, C. & Hildenbrand, K. An Al–Al one-electron π bond. Angew. Chem. Int. Ed. 32, 388–390 (1993).
Wright, R. J., Brynda, M. & Power, P. P. Synthesis and structure of the “dialuminyne” Na2[Ar’AlAlAr’] and Na2[(Ar“Al)3]: Al-Al bonding in Al2Na2 and Al3Na2 clusters. Angew. Chem. Int. Ed. 45, 5953–5956 (2006).
Franz, D. & Inoue, S. Advances in the development of complexes that contain a group 13 element chalcogen multiple bond. Dalton Trans. 45, 9385–9397 (2016).
Chu, T., Vyboishchikov, S. F., Gabidullin, B. & Nikonov, G. I. Oxidative cleavage of C=S and P=S bonds at an AlI center: preparation of terminally bound aluminum sulfides. Angew. Chem. Int. Ed. 55, 13306–13311 (2016).
Li, J. et al. Synthesis, structure, and reactivity of a monomeric iminoalane. Chem. Eur. J. 18, 15263–15266 (2012).
Hicks, J., Vasko, P., Goicoechea, J. M. & Aldridge, S. Synthesis, structure and reaction chemistry of a nucleophilic aluminyl anion. Nature 557, 92–95 (2018).
Hicks, J., Vasko, P., Goicoechea, J. M. & Aldridge, S. The aluminyl anion: a new generation of aluminium nucleophile. Angew. Chem. Int. Ed. 60, 1702–1713 (2021).
Anker, M. D. & Coles, M. P. Aluminium‐mediated carbon dioxide reduction by an isolated monoalumoxane anion. Angew. Chem. 131, 18429–18433 (2019).
Anker, M. D. & Coles, M. P. Isoelectronic aluminium analogues of carbonyl and dioxirane moieties. Angew. Chem. Int. Ed. 58, 13452–13455 (2019).
Anker, M. D., Schwamm, R. J. & Coles, M. P. Synthesis and reactivity of a terminal aluminium-imide bond. Chem. Commun. 56, 2288–2291 (2020).
Evans, M. J., Anker, M. D., McMullin, C. L., Rajabi, N. A. & Coles, M. P. Double insertion of CO2 into an Al-Te multiple bond. Chem. Commun. 57, 2673–2676 (2021).
Evans, M. J. et al. Carbon-chalcogen bond formation initiated by [Al(NONDipp)(E)]- anions containing Al-E{16} (E{16} = S, Se) multiple bonds. Chem. Sci. 13, 4635–4646 (2022).
Heilmann, A., Hicks, J., Vasko, P., Goicoechea, J. M. & Aldridge, S. Carbon monoxide activation by a molecular aluminium imide: C–O bond cleavage and C–C bond formation. Angew. Chem. Int. Ed. 59, 4897–4901 (2020).
Hicks, J., Heilmann, A., Vasko, P., Goicoechea, J. M. & Aldridge, S. Trapping and reactivity of a molecular aluminium oxide ion. Angew. Chem. Int. Ed. 58, 17265–17268 (2019).
Queen, J. D., Irvankoski, S., Fettinger, J. C., Tuononen, H. M. & Power, P. P. A monomeric aluminum imide (iminoalane) with Al–N triple-bonding: bonding analysis and dispersion energy stabilization. J. Am. Chem. Soc. 143, 6351–6356 (2021).
Hofmann, A., Légaré, M.-A., Wüst, L. & Braunschweig, H. Heterodiatomic multiple bonding in group 13: A complex with a boron-aluminium π bond reduces CO2. Angew. Chem. Int. Ed. 58, 9776–9781 (2019).
Fischer, M. et al. Isolable Phopha- and arsaalumenes. J. Am. Chem. Soc. 143, 4106–4111 (2021).
Schleyer, P. V. R. & Kost, D. A comparison of the energies of double bonds of second-row elements with carbon and silicon. J. Am. Chem. Soc. 110, 2105–2109 (1988).
Sakai, S. A theoretical investigation of Ziegler-Natta polymerization reaction mechanisms of acetylene. J. Phys. Chem. 95, 7089–7093 (1991).
Yan, C. & Kinjo, R. A three-membered diazo-aluminum heterocycle to access an Al=C π bonding species. Angew. Chem. Int. Ed. 61, e202211800 (2022).
Pelter, A., Singaram, B., Warren, L. & Wilson, J. W. Hindered organoboron groups in organic chemistry. 18. The production of boron stabilised carbanions. Tetrahedron 49, 2965–2978 (1993).
Maza, R. J., Carbó, J. J. & Fernández, E. Borata‐alkene species as nucleophilic reservoir. Adv. Synth. Catal. 363, 2274–2289 (2021).
Cuenca, A. B. & Fernández, E. Boron-Wittig olefination with gem-bis(boryl)alkanes. Chem. Soc. Rev. 50, 72–86 (2021).
Agarwal, A. & Bose, S. K. Bonding relationship between silicon and germanium with group 13 and heavier elements of groups 14–16. Chem. Asian J. 15, 3784–3806 (2020).
Suzuki, Y., Ishida, S., Sato, S., Isobe, H. & Iwamoto, T. An isolable potassium salt of a borasilene-chloride adduct. Angew. Chem. Int. Ed. 56, 4593–4597 (2017).
Nakata, N. & Sekiguchi, A. A stable silaborene: synthesis and characterization. J. Am. Chem. Soc. 128, 422–423 (2006).
Kajiwara, T., Takeda, N., Sasamori, T. & Tokitoh, N. Synthesis of alkali metal salts of borylsilyl anions utilizing highly crowded silylboranes and their properties. Organometallics 27, 880–893 (2008).
Wendel, D. et al. Silicon and oxygen’s bond of affection: an acyclic three-coordinate silanone and its transformation to an iminosiloxysilylene. J. Am. Chem. Soc. 139, 17193–17198 (2017).
Wiberg, N. et al. Supersilylsilane R*SiX3: Darstellung, Charakterisierung und Strukturen; sterische und van-der-Waals Effekte der Substituenten X [1] / Supersilylsilanes R*SiX3: Syntheses, characterization and structures; steric and van-der-Waals effects of substituents X [1]. Z. Naturforsch. B 55, 389–405 (2000).
Wiberg, N. et al. Disupersilylsilane R*2, Disupersilyldisilane R*2XSi-SiX3 und Tetrasupersilyltetrasilane R*2XSi-SiX2-SiX2-SiXR*2. Z. Anorg. Allg. Chem. 627, 594–606 (2001).
Sekiguchi, A., Fukawa, T., Nakamoto, M., Lee, V. Y. & Ichinohe, M. Isolable silyl and germyl radicals lacking conjugation with pi-bonds: synthesis, characterization, and reactivity. J. Am. Chem. Soc. 124, 9865–9869 (2002).
Sänger, I. et al. The inverse sandwich complex (K(18-crown-6))2CpCpFe(CO)2 -unpredictable redox reactions of [CpFe(CO)2]I with the silanides Na[SiRtBu2] (R = Me, tBu) and the isoelectronic phosphanyl borohydride K[PtBu2BH3]. Dalton Trans. 41, 6671–6676 (2012).
Klinkhammer, K. W. Tris(trimethylsilyl)silanides of the heavier alkali metals—a structural study. Chem. Eur. J. 3, 1418–1431 (1997).
Lerner, H.-W., Scholz, S. & Bolte, M. Synthese, Struktur und Eigenschaften des Natriumsilanids tBu2PhSiNa - ein Zugang zu Di-tert-butyl-phenylsilylsubstituierten Verbindungen. Z. Anorg. Allg. Chem. 627, 1638–1642 (2001).
Engesser, T. A., Lichtenthaler, M. R., Schleep, M. & Krossing, I. Reactive p-block cations stabilized by weakly coordinating anions. Chem. Soc. Rev. 45, 789–899 (2016).
Nakamoto, M., Shimizu, K. & Sekiguchi, A. Two-coordinate group 13 element (Al, Ga) centered cations formed by silyl group migration: synthesis and characterization. Chem. Lett. 36, 984–985 (2007).
Wochele, R., Schwarz, W., Klinkhammer, K. W., Locke, K. & Weidlein, J. Neue Hypersilanide der Erdmetalle Aluminium, Gallium und Indium. Z. Anorg. Allg. Chem. 626, 1963–1973 (2000).
Wiberg, N., Blank, T., Kaim, W., Schwederski, B. & Linti, G. Tri(supersilyl)dialanyl (tBu3Si)3Al2• and tetra(supersilyl)cyclotrialanyl (tBu3Si)4Al3• − new stable radicals of a group 13 element from thermolysis of (tBu3Si)4Al2. Eur. J. Inorg. Chem. 2000, 1475–1481 (2000).
Wiberg, N. et al. Supersilyltrielane RnEHal3–n (E = Triel, R = SitBu3): Synthesen, Charakterisierung, Reaktionen, Strukturen [1] / Supersilyltrielanes RnEHal3–n (E = Triel, R = SitBu3): syntheses, characterization, reactions, structures [1]. Z. Naturforsch. B 56, 634–651 (2001).
Hill, M. S., Kociok-Köhn, G. & MacDougall, D. J. N-Heterocyclic carbenes and charge separation in heterometallic s-block silylamides. Inorg. Chem. 50, 5234–5241 (2011).
Su, J., Li, X.-W., Crittendon, R. C. & Robinson, G. H. How short is a -Ga⋮Ga- triple bond? synthesis and molecular structure of Na2[Mes*2C6H3-Ga⋮Ga-C6H3Mes*2] (Mes* = 2,4,6-i-Pr3C6H2): the first gallyne. J. Am. Chem. Soc. 119, 5471–5472 (1997).
López, C. S., Nieto Faza, O., Cossío, F. P., York, D. M. & de Lera, A. R. Ellipticity: a convenient tool to characterize electrocyclic reactions. Chem. Eur. J. 11, 1734–1738 (2005).
Cabeza, J. A., van der Maelen, J. F. & García-Granda, S. Topological analysis of the electron density in the N-heterocyclic carbene triruthenium cluster [Ru3(μ-H)2(μ3-MeImCH)(CO)9] (Me2Im = 1,3-dimethylimidazol-2-ylidene). Organometallics 28, 3666–3672 (2009).
Shaik, S., Danovich, D., Wu, W. & Hiberty, P. C. Charge-shift bonding and its manifestations in chemistry. Nat. Chem. 1, 443–449 (2009).
Popelier, P. On the full topology of the Laplacian of the electron density. Coord. Chem. Rev. 197, 169–189 (2000).
Power, P. P. An update on multiple bonding between heavier main group elements: the importance of Pauli repulsion, charge-shift character, and London dispersion force effects. Organometallics 39, 4127–4138 (2020).
Yanagisawa, T., Mizuhata, Y. & Tokitoh, N. Syntheses and structures of novel λ3,λ3-phosphanylalumanes fully bearing carbon substituents and their substituent effects. Inorganics 7, 132 (2019).
Kuhn, N. & Kratz, T. Synthesis of imidazol-2-ylidenes by reduction of imidazole-2-(3H)-thiones. Synthesis 1993, 561–562 (1993).
Arduengo, A. J., Dias, H. V. R., Harlow, R. L. & Kline, M. Electronic stabilization of nucleophilic carbenes. J. Am. Chem. Soc. 114, 5530–5534 (1992).
APEX 3 v.2015.5-2 (Bruker AXS, 2015).
Neese, F. Software update: the ORCA program system, version 4.0. WIREs Comput. Mol. Sci. 8, e1327 (2017).
Becke, A. D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 98, 5648–5652 (1993).
Lee, C., Yang, W. & Parr, R. G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 37, 785–789 (1988).
Schäfer, A., Huber, C. & Ahlrichs, R. Fully optimized contracted Gaussian basis sets of triple zeta valence quality for atoms Li to Kr. J. Chem. Phys. 100, 5829–5835 (1994).
Weigend, F. & Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 7, 3297–3305 (2005).
Efficient, approximate and parallel Hartree–Fock and hybrid DFT calculations. A ‘chain-of-spheres’ algorithm for the Hartree–Fock exchange. Chem. Phys. 356, 98–109 (2009).
Caldeweyher, E. et al. A generally applicable atomic-charge dependent London dispersion correction. J. Chem. Phys. 150, 154122 (2019).
Caldeweyher, E., Bannwarth, C. & Grimme, S. Extension of the D3 dispersion coefficient model. J. Chem. Phys. 147, 34112 (2017).
Cossi, M., Rega, N., Scalmani, G. & Barone, V. Energies, structures, and electronic properties of molecules in solution with the C-PCM solvation model. J. Comp. Chem. 24, 669–681 (2003).
Barone, V. & Cossi, M. Quantum calculation of molecular energies and energy gradients in solution by a conductor solvent model. J. Chem. Phys. A 102, 1995–2001 (1998).
Ishida, K., Morokuma, K. & Komornicki, A. The intrinsic reaction coordinate. An ab initio calculation for HNC → HCN and H− + CH4 → CH4 + H−. J. Chem. Phys. 66, 2153–2156 (1977).
Riplinger, C., Sandhoefer, B., Hansen, A. & Neese, F. Natural triple excitations in local coupled cluster calculations with pair natural orbitals. J. Chem. Phys. 139, 134101 (2013).
Pople, J. A., Head-Gordon, M. & Raghavachari, K. Quadratic configuration interaction. A general technique for determining electron correlation energies. J. Chem. Phys. 87, 5968 (1987).
Schreckenbach, G. & Ziegler, T. Calculation of NMR shielding tensors using gauge-including atomic orbitals and modern density functional theory. J. Chem. Phys. 99, 606–611 (1995).
Gese, A. et al. Toward a 1,4-diphosphinine-based molecular CPS-ternary compound. Inorg. Chem. 60, 13029–13040 (2021).
Acknowledgements
We acknowledge K. Kollmannsberger (R. A. Fischer) for the IR measurements. We thank R. Ebner for assistance with initial reactivity studies and M. Roy for proofreading. A.E.F. thanks the Servicio de Cálculo Científico at the University of Murcia for technical support and computational resources used. The authors are grateful to the European Research Council (ALLOWE101001591) for financial support. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
M.L. and D.F. conceived and performed the synthetic experiments and analysed the data. A.E.F. designed and performed the theoretical analyses. M.B. and F.H. solved and revised the XRD data. S.I. conceived and supervised the project. M.L., D.F. and S.I. wrote the manuscript with input and critical revision from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks David Scheschkewitz, Petra Vasko and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–69, Tables 1–6, synthetic procedures for all compounds, crystallographic and computational details.
Supplementary Data 1
Crystallographic data for compound 2·IMes, CCDC 2170414.
Supplementary Data 2
Crystallographic data for compound 3·NMe3, CCDC 2170415.
Supplementary Data 3
Crystallographic data for compound 4·NMe3, CCDC 2170416.
Supplementary Data 4
Crystallographic data for compound 5·Na(15-c-5), CCDC 2170417.
Supplementary Data 5
Crystallographic data for compound 5·NaIiPr, CCDC 2170418.
Supplementary Data 6
Crystallographic data for compound Na·crypt[5], CCDC 2170419.
Supplementary Data 7
Crystallographic data for compound Na·(DME)3[6], CCDC 2170420.
Supplementary Data 8
Crystallographic data for compound 7, CCDC 2170421.
Supplementary Data 9
Source data for Supplementary Fig. 67.
Supplementary Data 10
Cartesian coordinates of optimized and calculated structures, electronic energies for calculated structures.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ludwig, M., Franz, D., Espinosa Ferao, A. et al. Anions featuring an aluminium–silicon core with alumanyl silanide and aluminata-silene characteristics. Nat. Chem. 15, 1452–1460 (2023). https://doi.org/10.1038/s41557-023-01265-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-023-01265-3