Abstract
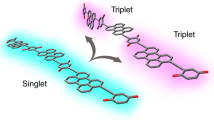
Quantum interference (QI)—the constructive or destructive interference of conduction pathways through molecular orbitals—plays a fundamental role in enhancing or suppressing charge and spin transport in organic molecular electronics. Graphical models were developed to predict constructive versus destructive interference in polyaromatic hydrocarbons and have successfully estimated the large conductivity differences observed in single-molecule transport measurements. A major challenge lies in extending these models to excitonic (photoexcited) processes, which typically involve distinct orbitals with different symmetries. Here we investigate how QI models can be applied as bridging moieties in intramolecular singlet-fission compounds to predict relative rates of triplet pair formation. In a series of bridged intramolecular singlet-fission dimers, we found that destructive QI always leads to a slower triplet pair formation across different bridge lengths and geometries. A combined experimental and theoretical approach reveals the critical considerations of bridge topology and frontier molecular orbital energies in applying QI conductance principles to predict rates of multiexciton generation.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The raw transient absorption data used to generate Figs. 2 and 3 and the corresponding analysis are freely available via the Dryad public repository: https://doi.org/10.5061/dryad.x95x69pnn. Additional data are available upon request. Source data are provided with this paper.
References
Su, T. A., Neupane, M., Steigerwald, M. L., Venkataraman, L. & Nuckolls, C. Chemical principles of single-molecule electronics. Nat. Rev. Mater. 1, 16002 (2016).
Lambert, C. J. Basic concepts of quantum interference and electron transport in single-molecule electronics. Chem. Soc. Rev. 44, 875–888 (2015).
Jan van der Molen, S. & Liljeroth, P. Charge transport through molecular switches. J. Phys. Condens. Matter 22, 133001 (2010).
Guédon, C. M. et al. Observation of quantum interference in molecular charge transport. Nat. Nanotechnol. 7, 305–309 (2012).
Arroyo, C. R. et al. Signatures of quantum interference effects on charge transport through a single benzene ring. Angew. Chem. Int. Ed. 52, 3152–3155 (2013).
Solomon, G. C. et al. Understanding quantum interference in coherent molecular conduction. J. Chem. Phys. 129, 054701 (2008).
Tsuji, Y., Hoffmann, R., Strange, M. & Solomon, G. C. Close relation between quantum interference in molecular conductance and diradical existence. Proc. Natl Acad. Sci. USA 113, E413–E419 (2016).
Markussen, T., Stadler, R. & Thygesen, K. S. The relation between structure and quantum interference in single molecule junctions. Nano Lett. 10, 4260–4265 (2010).
Ortiz, R. et al. Exchange rules for diradical π-conjugated hydrocarbons. Nano Lett. 19, 5991–5997 (2019).
Gorczak, N. et al. Computational design of donor–bridge–acceptor systems exhibiting pronounced quantum interference effects. Phys. Chem. Chem. Phys. 18, 6773–6779 (2016).
Gorczak, N. et al. Charge transfer versus molecular conductance: molecular orbital symmetry turns quantum interference rules upside down. Chem. Sci. 6, 4196–4206 (2015).
Sanders, S. N. et al. Exciton correlations in intramolecular singlet fission. J. Am. Chem. Soc. 138, 7289–7297 (2016).
Pun, A. B. et al. Ultra-fast intramolecular singlet fission to persistent multiexcitons by molecular design. Nat. Chem. 11, 821–828 (2019).
Krishnapriya, K. C. et al. Spin density encodes intramolecular singlet exciton fission in pentacene dimers. Nat. Commun. 10, 33 (2019).
Hetzer, C., Guldi, D. M. & Tykwinski, R. R. Pentacene dimers as a critical tool for the investigation of intramolecular singlet fission. Chem. Eur. J. 24, 8245–8257 (2018).
Basel, B. S. et al. Influence of the heavy-atom effect on singlet fission: a study of platinum-bridged pentacene dimers. Chem. Sci. 10, 11130–11140 (2019).
Margulies, E. A. et al. Enabling singlet fission by controlling intramolecular charge transfer in π-stacked covalent terrylenediimide dimers. Nat. Chem. 8, 1120–1125 (2016).
Sakuma, T. et al. Long-lived triplet excited states of bent-shaped pentacene dimers by intramolecular singlet fission. J. Phys. Chem. A 120, 1867–1875 (2016).
Nakamura, S. et al. Enthalpy–entropy compensation effect for triplet pair dissociation of intramolecular singlet fission in phenylene spacer-bridged hexacene dimers. J. Phys. Chem. Lett. 12, 6457–6463 (2021).
Korovina, N. V. et al. Linker-dependent singlet fission in tetracene dimers. J. Am. Chem. Soc. 140, 10179–10190 (2018).
Ito, S., Nagami, T. & Nakano, M. Design principles of electronic couplings for intramolecular singlet fission in covalently-linked systems. J. Phys. Chem. A 120, 6236–6241 (2016).
Paul, S., Govind, C. & Karunakaran, V. Planarity and length of the bridge control rate and efficiency of intramolecular singlet fission in pentacene dimers. J. Phys. Chem. B 125, 231–239 (2021).
Abraham, V. & Mayhall, N. J. Revealing the contest between triplet–triplet exchange and triplet–triplet energy transfer coupling in correlated triplet pair states in singlet fission. J. Phys. Chem. Lett. 12, 10505–10514 (2021).
Abraham, V. & Mayhall, N. J. Simple rule to predict boundedness of multiexciton states in covalently linked singlet-fission dimers. J. Phys. Chem. Lett. 8, 5472–5478 (2017).
Chesler, R., Khan, S. & Mazumdar, S. Wave function based analysis of dynamics versus yield of free triplets in intramolecular singlet fission. J. Phys. Chem. A 124, 10091–10099 (2020).
Herrmann, C. Electronic communication as a transferable property of molecular bridges? J. Phys. Chem. A 123, 10205–10223 (2019).
Walter, D., Neuhauser, D. & Baer, R. Quantum interference in polycyclic hydrocarbon molecular wires. Chem. Phys. 299, 139–145 (2004).
Bajaj, A., Kaur, P., Sud, A., Berritta, M. & Ali, Md. E. Anomalous effect of quantum interference in organic spin filters. J. Phys. Chem. C 124, 24361–24371 (2020).
Sanders, S. N. et al. Quantitative intramolecular singlet fission in bipentacenes. J. Am. Chem. Soc. 137, 8965–8972 (2015).
Parenti, K. R. et al. Bridge resonance effects in singlet fission. J. Phys. Chem. A 124, 9392–9399 (2020).
Zirzlmeier, J. et al. Singlet fission in pentacene dimers. Proc. Natl Acad. Sci. USA 112, 5325–5330 (2015).
Korovina, N. V., Pompetti, N. F. & Johnson, J. C. Lessons from intramolecular singlet fission with covalently bound chromophores. J. Chem. Phys. 152, 040904 (2020).
Davis, W. B., Svec, W. A., Ratner, M. A. & Wasielewski, M. R. Molecular-wire behaviour in p -phenylenevinylene oligomers. Nature 396, 60–63 (1998).
Fuemmeler, E. G. et al. A direct mechanism of ultrafast intramolecular singlet fission in pentacene dimers. ACS Cent. Sci. 2, 316–324 (2016).
Yablon, L. M. et al. Singlet fission and triplet pair recombination in bipentacenes with a twist. Mater. Horiz. 9, 462–470 (2022).
Quinn, J. R., Foss, F. W., Venkataraman, L., Hybertsen, M. S. & Breslow, R. Single-molecule junction conductance through diaminoacenes. J. Am. Chem. Soc. 129, 6714–6715 (2007).
Pariser, R. & Parr, R. G. A semi‐empirical theory of the electronic spectra and electronic structure of complex unsaturated molecules. I. J. Chem. Phys. 21, 466–471 (1953).
Pople, J. A. Electron interaction in unsaturated hydrocarbons. Trans. Faraday Soc. 49, 1375 (1953).
Smith, M. B. & Michl, J. Recent advances in singlet fission. Annu. Rev. Phys. Chem. 64, 361–386 (2013).
Berkelbach, T. C., Hybertsen, M. S. & Reichman, D. R. Microscopic theory of singlet exciton fission. II. Application to pentacene dimers and the role of superexchange. J. Chem. Phys. 138, 114103 (2013).
Khan, S. & Mazumdar, S. Diagrammatic exciton basis theory of the photophysics of pentacene dimers. J. Phys. Chem. Lett. 8, 4468–4478 (2017).
Khan, S. & Mazumdar, S. Theory of transient excited state absorptions in pentacene and derivatives: triplet–triplet biexciton versus free triplets. J. Phys. Chem. Lett. 8, 5943–5948 (2017).
Kittel, C. & Fong, C. Y. Quantum Theory of Solids (Wiley, 1987).
Hele, T. J. H. et al. Anticipating acene-based chromophore spectra with molecular orbital arguments. J. Phys. Chem. A 123, 2527–2536 (2019).
Beljonne, D., Yamagata, H., Brédas, J. L., Spano, F. C. & Olivier, Y. Charge-transfer excitations steer the Davydov splitting and mediate singlet exciton fission in pentacene. Phys. Rev. Lett. 110, 226402 (2013).
Basel, B. S. et al. Evidence for charge-transfer mediation in the primary events of singlet fission in a weakly coupled pentacene dimer. Chem 4, 1092–1111 (2018).
Busby, E. et al. A design strategy for intramolecular singlet fission mediated by charge-transfer states in donor–acceptor organic materials. Nat. Mater. 14, 426–433 (2015).
He, G. et al. Charge transfer states impact the triplet pair dynamics of singlet fission polymers. J. Chem. Phys. 153, 244902 (2020).
Monahan, N. & Zhu, X.-Y. Charge transfer-mediated singlet fission. Annu. Rev. Phys. Chem. 66, 601–618 (2015).
Tsuji, Y. & Yoshizawa, K. Frontier orbital perspective for quantum interference in alternant and nonalternant hydrocarbons. J. Phys. Chem. C 121, 9621–9626 (2017).
Xia, J. et al. Breakdown of interference rules in azulene, a nonalternant hydrocarbon. Nano Lett. 14, 2941–2945 (2014).
Kumarasamy, E. et al. Tuning singlet fission in π-bridge-π chromophores. J. Am. Chem. Soc. 139, 12488–12494 (2017).
Aryanpour, K., Shukla, A. & Mazumdar, S. Theory of singlet fission in polyenes, acene crystals, and covalently linked acene dimers. J. Phys. Chem. C 119, 6966–6979 (2015).
Ramasesha, S., Albert, I. D. L. & Sinha, B. Optical and magnetic properties of the exact PPP states of biphenyl. Mol. Phys. 72, 537–547 (1991).
Khan, S. & Mazumdar, S. Optical probes of the quantum-entangled triple–triplet state in a heteroacene dimer. Phys. Rev. B 98, 165202 (2018).
Chandross, M. & Mazumdar, S. Coulomb interactions and linear, nonlinear, and triplet absorption in poly(para-phenylenevinylene). Phys. Rev. B 55, 1497–1504 (1997).
Tavan, P. & Schulten, K. Electronic excitations in finite and infinite polyenes. Phys. Rev. B 36, 4337–4358 (1987).
Acknowledgements
This work was supported by the US Department of Energy, Office of Science, Office of Basic Energy Sciences under award no. DE-SC0022036 (M.Y.S. and L.M.C.) and the National Science Foundation under award no. CHE-1764152 (S.M.). K.R.P. thanks the Department of Defense for a National Defense Science and Engineering (NDSEG) Fellowship. J.Z. thanks the Columbia College Science Scholars Program and Guthikonda Fellowship. X.Y. acknowledges the Beijing Institute of Technology Research Fund Program for Young Scholars, and the Analysis and Testing Center of Beijing Institute of Technology for NMR and mass spectrometry characterization. This research used resources at the Center for Functional Nanomaterials, which is a US DOE Office of Science Facility at Brookhaven National Laboratory under contract DE-SC0012704.
Author information
Authors and Affiliations
Contributions
S.M., M.Y.S and L.M.C. oversaw the project. K.R.P., M.Y.S. and L.M.C. designed the molecules. G.H. collected the transient absorption spectroscopy data and K.R.P., G.H. and M.Y.S. carried out data analysis. K.R.P., B.X., H.H., D.M. and J.Z. synthesized and characterized the molecules, supervised by X.Y. and L.M.C. The theory models were designed by S.M. and A.S. PPP calculations were carried out by R.C. and P.B. Density functional theory calculations were carried out by G.H. The paper was written by K.R.P., S.M., M.Y.S. and L.M.C. with contributions from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Peer review
Peer review information
Nature Chemistry thanks Ferdinand Grozema and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Density functional theory geometry calculations.
The ground state geometry of P–15N–P and P–16N–P are optimized using density functional theory (DFT) at the B3LYP/6-31G(d) level. Substitution at the 2, 6 and 7 positions of naphthalene gives a dihedral angle of ~35°, consistent with previous calculations on acene dimers10. Substitution at the 1 and 5 positions of naphthalene gives a more distorted structure with a dihedral angle of ~58°. As has been previously shown, singlet fission slows down considerably in highly twisted dimers10,11. We show the geometry of the twisted dimers and summarize all the naphthalene substitution positions and calculated dihedral angles in Extended Data Fig. 2.
Extended Data Fig. 2
Naphthalene substitution and corresponding dihedral angles calculated with DFT.
Extended Data Fig. 3 Hückel energy level distribution for T–N–T displaying the active space of 24 MOs.
The MRSDCI calculations are over an active space of MOs about the chemical potential that is smaller than the complete set. The lowest few bonding MOs are frozen (that is, excitations from these lowest MOs are not included in the CI calculations) and the highest MOs related to these by charge-conjugation symmetry are excluded from the active space. As of now, we have performed MRSDCI calculations over active spaces of 22–30 MOs, 11–15 bonding and 11–15 antibonding. These are absolutely the largest active spaces over which calculations of \(^1\left| {TT} \right\rangle\) have ever been done. We illustrate our procedure with the specific case of T–N–T. The localized tetracene Hückel bonding (red) and antibonding (blue) MOs, and the naphthalene (green) MOs constitute the active MO space in our T–N–T calculations. For calculations on T–Ph–T, T–A–T, P–Ph–P, P–N–P and P–A–P, we retained 20 + 4, 16 + 6, 20 + 4, 20 + 6 and 20 + 6 MOs, respectively, where the numbers in each sum indicate the chromophore MOs plus the linker MOs.
Supplementary information
Supplementary Information
Supplementary Figs. 1–12, discussion and Table 1.
Source data
Source Data Extended Data Fig. 2
Naphthalene substitution and corresponding dihedral angles calculated with DFT.
Source Data Extended Data Fig. 4
Time constants for singlet fission, triplet pair decay, and free triplet decay. Uncertainties are +/− 5%.
Rights and permissions
About this article
Cite this article
Parenti, K.R., Chesler, R., He, G. et al. Quantum interference effects elucidate triplet-pair formation dynamics in intramolecular singlet-fission molecules. Nat. Chem. 15, 339–346 (2023). https://doi.org/10.1038/s41557-022-01107-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-022-01107-8