Abstract
Molecules that contain one or more fluorine atoms are crucial to drug discovery. There are protocols available for the selective synthesis of different organofluorine compounds, including those with a fluoro-substituted or a trifluoromethyl-substituted stereogenic carbon centre. However, approaches for synthesizing compounds with a trifluoromethyl- and fluoro-substituent stereogenic carbon centre are far less common. This potentially impactful set of molecules thus remains severely underdeveloped. Here we introduce a catalytic regio-, diastereo- and enantioselective strategy for the preparation of homoallylic alcohols bearing a stereogenic carbon centre bound to a trifluoromethyl group and a fluorine atom. The process, which involves a polyfluoroallyl boronate and is catalysed by an in situ-formed organozinc complex, can be used for diastereodivergent preparation of tetrafluoro-monosaccharides, including ribose core analogues of the antiviral drug sofosbuvir (Sovaldi). Unexpected reactivity/selectivity profiles, probably originating from the trifluoromethyl- and fluoro-substituted carbon site, are discovered, foreshadowing other unique chemistries that remain unknown.

Similar content being viewed by others
Main
The ease, economy, efficiency and selectivity with which organofluorine compounds are accessed is in the exclusive purview of chemical synthesis1,2. Efficient transformations that deliver valuable fluoro-organic products with high diastereo- and/or enantioselectivity open fresh vistas in drug discovery3,4,5, and facilitate the development of improved agrochemicals6 and/or superior polymeric materials7. Among the areas to be impacted are oligonucleotide therapeutics and glycomimetic drug design8,9,10,11, where 2-fluoro-substituted monosaccharides are key (Fig. 1a). An example is sofosbuvir, sold under the name Sovaldi, which is used for the treatment of chronic hepatitis C virus infection12,13,14,15. A more potent derivative of Sovaldi has a fluoro,bromo-substituted stereogenic C2 (ref. 16). Bioactive pyranosides with a fluoro-substituted C2 are similarly sought-after, a prominent member being sialyltransferase inhibitor 3Fax-Neu5Ac17,18. These latter compounds are components of cancer vaccine candidates19,20 that can be used discretely, or in combination with other drugs, to counter viral infections21, including COVID-19 (refs. 22,23,24).
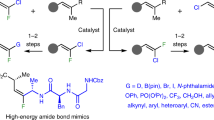
a, Key polyfluoro nucleosides include gemcitabine (sold as Gemzar) and sofosbuvir (sold as Sovaldi). Analogues bearing a fluoro- and trifluoromethyl-substituted C2 are of considerable interest, but remain largely unexplored. b, Our aim was to establish a strategy for diastereodivergent and enantioselective synthesis of trifluoromethyl- and fluoro-substituted monosaccharides. This would involve two modes of stereoselective additions of trifluoromethyl- and fluoro-substituted allylboronates E- and/or Z-1a to aldehyde 2. There was precedent for one pathway, affording R,R,R-3a, but none for accessing R,R,S-3a. It remained to be seen if a catalyst-controlled method would address this problem and if a concise sequence might be developed for synthesizing the targeted heterocycles. c, It was initially projected that considerable amounts of α-addition products might be generated and that the main component of the γ-addition route would be the isomer shown here. Intriguingly, this turned out not to be the case. G, R, a functional group or chain; pin, pinacolato; pg, protecting group; L, ligand(s).
In light of evidence vis-à-vis the beneficial impact of a trifluoromethyl group on bioavailability and/or metabolic stability of a therapeutic candidate2,3, the development of efficient and stereoselective pathways for the synthesis of unexplored furanosides and pyranosides with a trifluoromethyl- and fluoro-substituted C2 (refs. 2,25) is particularly desirable (Fig. 1a). Oxonium ion generation and the ensuing saccharide ring cleavage, a preamble to depurination26,27, might then be thwarted by the strong electronic pull caused by the trifluoromethyl- and fluoro-substituted stereogenic carbon (compared to those with a fluorine-substituted stereogenic centre). Although a fluoro-nucleotide can mimic RNA, the fully substituted carbon offsets C–O elimination and backbone cleavage by proton removal28,29, consistent with an additional heteroatomic moiety at C2 enhancing potency (for example, the 2′-dihalo ribonucleotide prodrugs active against hepatitis C virus16,30). Furthermore, four relatively short C–F bonds and strongly electron-withdrawing fluorine atoms, together with 12 pairs of non-bonding electrons within a confined space, can elicit unique reactivity and/or selectivity profiles.
Catalytic methods have been introduced for the enantioselective synthesis of compounds with a fluoro-substituted31 or a trifluoromethyl-substituted quaternary carbon stereogenic centre32,33,34. By contrast, there are only two approaches pertaining to those containing a fully substituted stereogenic carbon centre with a trifluoromethyl group and a fluorine atom. One entails reaction of a 7-azaindoline amide with an aryl- or heteroaryl-substituted Boc-imide (Boc, tert-butyloxycarbonyl)35, used to synthesize a fluoro-analogue of a T-type selective Ca2+-channel blocker36 and a fluorinated bioisostere of isoleucine37. The other is for enantioselective addition of two fluorine atoms to trifluoromethyl-substituted aryl olefins, furnishing products with a fluoro-, monofluoromethyl- and trifluoromethyl-substituted carbon centre38,39. These advances are noteworthy but do not easily lend themselves to nucleosides synthesis.
Results and discussion
Preliminary considerations
An attractive option for the core transformation would be the addition of trifluoromethyl- and fluoro-substituted allylboronates Z-1a and/or E-1a (Fig. 1b) to aldehydes. Stereospecific reactions involving trifluoromethyl-fluoro-substituted allylboronate 1a and aldehyde 2, by substrate- or catalyst-controlled routes, could yield homoallylic alcohols R,R,R-3a or R,R,S-3a. Subsequent modification, including protecting-group adjustments, would generate hydroxy-aldehydes I–IV, precursors to furanoses V and VII or pyranoses VI and VIII (Fig. 1b). For a substrate-controlled approach, a method developed by Aggarwal40 could be adopted. We surmised that R,R,R-3a could serve as precursor to monosaccharides V and VI. To prepare the alternative diastereomer, R,R,S-3a, on the other hand, we would need a catalytic strategy for γ- and diastereoselective addition of allylboronate 1a to aldehyde 2. Moreover, for application to the synthesis of polyfluoro nucleosides, reactions with enolizable aliphatic aldehydes would have to be efficient.
A suitable catalytic transformation
Regarding a catalyst-controlled process, we favoured the chiral aminophenol-derived system41 (Fig. 1c). Accessibility, ease of modification and robustness aside, previous studies show that initial outcomes may be optimized based on mechanistic analysis. For example, rationales have been developed for why certain additions are exceedingly α-selective42,43 whereas others afford the γ-addition product preferentially44. We anticipated a similar scenario for a trifluoromethyl- and fluoro-substituted allylboronate, namely, that the initially formed complex i (Fig. 1c) would react with an aldehyde via ts-i. We expected needing to devise ways to improve γ:α ratios, and envisioned doing so by lowering the rate of the addition of the initially formed allylboronate i and/or by accelerating 1,3-boryl shift44 (i → ii). We were additionally concerned that reaction via ii and ts-ii would generate the same diastereomer as that formed by a substrate-controlled process40.
Unanticipated regio- and diastereoselectivity
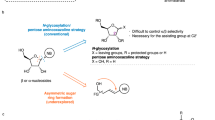
Polyfluoro allylboronate 1a (Table 1) was prepared in multi-gram quantities (63% yield, 98:2 Z:E)45,46, and its reaction with benzaldehyde in the presence of a range of aminophenols was investigated (see Supplementary Information part 1, section 8.1.1, for details). We found that, with 5.0 mol% of a commercially available aminophenol (ap), 13 mol% Zn(Ot-Bu)Et, and 5.0 equiv. of MeOH, the transformation proceeds smoothly at 60 °C, affording—to our surprise—the γ-addition isomer, S,R-3b, preferentially (87%, compared to 13% α-addition isomer 4) and in 85:15 diastereomeric ratio (d.r.). Upon purification, S,R-3b was isolated in 59% yield, as a single regio- and diastereomer (>98:2 γ:α and d.r.) and in 94:6 enantiomeric ratio (e.r.). At ambient temperature, the transformation was similarly selective, but less efficient (for example, 76% conversion after 8 or 32 h at 22 °C).
We had so far made two observations that, although auspicious, were entirely unforeseen. One was the that the major diastereomer formed was initially predicted to be a minor component (Fig. 1c). The other was that, contrary to the reactions with trifluoromethyl-substituted allylboronates43, γ selectivity was high. Mechanistic revisions were clearly needed, but we chose to explore the scope and applicability of the catalytic method first. Although aldehydes such as 2 (Fig. 1b) are more relevant to monosaccharide synthesis, the development of a more general catalytic method would be equally important. The modifiability of homoallylic alcohols would deliver other desirable and otherwise difficult-to-access organofluorine compounds.
Scope of the method
Reactions with sterically and electronically distinct aryl- (3c–g) and heteroaryl-substituted (3h–j) aldehydes (Table 1) furnished products in 89:11 to >98:2 γ:α ratio. Homoallylic alcohols 3c–j were generated in 95:5 to >98:2 d.r. (pure γ, except 3e and 3j) and isolated in yields ranging from 34% for the relatively volatile 3e to 77% for furyl-substituted 3i. Enantioselectivities were generally high (89:11–96.5:3.5 e.r.).
Aldehyde electrophilicity impacts γ selectivity. The more electron-deficient probably react with a chiral allyl complex before allyl shift can occur (i → ii, Fig. 1c; see Figs. 3 and 4 for the revised mechanistic analysis) and, consequently, the γ:α ratios are lower (for example, 3g, 80% γ). Conversely, γ selectivity is higher for the more electron-rich aldehydes (for example, 3c and 3f, 93% and 95% γ, respectively). Alkenyl- and alkyl-substituted aldehydes may be used: 3k–m (pure γ) were isolated in 53–65% yield, 93:7 to >98:2 d.r. and 91:9–95:5 e.r. The transformation with an enantiomerically pure β-branched aldehyde afforded a 75:15:6:4 diastereomeric mixture, and 3n was isolated in 58% yield (pure γ) and >98:2 d.r. Reactions with Z-1b, an allylboronate derived from a commercially available nonafluoro-alkene, were also performed, illustrating that the corresponding perfluoro compounds may be accessed (5a,b). The X-ray structures for 3j and 5a confirmed the stereochemical assignments.
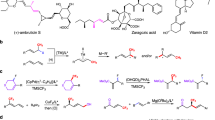
a, In the case of the more electrophilic aldehyde (2), a larger excess of MeOH leads to higher γ selectivity. This is attributed to an increase in hemiacetal formation and lower aldehyde concentration, allowing allyl isomerization to occur before C–C bond formation. b, Diastereo- and enantiomerically enriched homoallylic alcohol R,R,S-3a was thus converted to polyfluoro furanose R,R,S-7 and pyranose R,R,S-10. An unexpected observation was that reaction of R,R,S-7 with benzyl bromide led to preferential ether formation at the more hindered secondary alcohol. c, Substrate-controlled reaction of allylboronate 1a and 2 afforded R,R,R-3a, which was used to access polyfluoro furanose R,R,R-12 and pyranose R,R,R-13. Again, the more hindered secondary alcohol in R,R,R-8 reacted faster to afford R,R,R-9 preferentially. Reactions were performed under a N2 atmosphere. Conversion (aldehyde disappearance, >98% in all cases) and diastereoselectivity were determined by analysis of 19F or 1H NMR spectra of unpurified product mixtures (±2%). Yields are for pure products (±5%). Enantioselectivity was determined by HPLC analysis (±1%). See Supplementary Information part 1, section 6, for details. pin, pinacolato; NMO, N-methylmorpholine N-oxide; DMAP, 4-dimethylaminopyridine; p-TsOH, p-toluenesulfonic acid; Bz, benzoate; Bn, benzyl; CSA, camphorsulfonic acid; TFAA, trifluoroacetic anhydride; TBSCl, tert-butyldimethylsilyl chloride.
a, DFT studies indicate that allyl isomerization probably involves an organozinc, and not an organoboron, catalyst. The longer C…Zn distances (compared to C…B) help relieve steric and electron–electron repulsion, leading to lower transition-state energies. b, The efficiency and stereochemical outcome of a catalytic process does not depend on the stereoisomeric purity of allylboronate 1a, which is congruent with the involvement of ts-Zn-1 and ts-Zn-2 in the isomerization event. c, The X-ray structure of (ap)2Zn2 points to the viability of a Zn–aminophenol complex. DFT studies performed at the M06/6-311+G(d,p)-SDD,SMD(toluene)//B3LYP-D3/6-31G(d)-SDD level. See Supplementary Information part 2, section 2.1, for details. ts, transition state.
a, A catalytic cycle is proposed based on kinetic and DFT studies The turnover-limiting step is probably isomerization of the initially generated allylzinc complex via ts-Zn-1 or ts-Zn-2 to 14, which readily reacts with an aldehyde to generate the final product (S,R-3b) via ts-vi and v. The order in allylboronate 1a is +0.3, probably because it is an in situ-formed and less encumbered derivative, 1a′ and/or 1a″, which is the active species. The −0.6 kinetic order in MeOH may be a balance between the formation of 1a′ and 1a″ (positive) and the formation of Zn(OMe)2 and inactive iii′ (negative). b, Studies involving 1b show that the trifluoromethyl- and fluoro-substituted carbon are important to the outcome of a process. c, Investigations with 1c indicate that a trifluoromethyl- as well as a fluoro- substituent are needed for the favoured mode of regioselectivity. This might be due to a preference for ts-iii over ts-iii′ and ts-Zn-1 over ts-iv or ts-iv′, all of which are on account of the presence of the polyfluoro carbon. DFT studies performed at the M06/6-311+G(d,p)-SDD,SMD(toluene)//B3LYP-D3/6-31G(d)-SDD level. See Supplementary Information part 2, section 2.1, for details. ap, aminophenol; ts, transition state.
Synthesis of polyfluoro monosaccharides
Aldehyde 2 (Fig. 1b; >99:1 e.r.), although purchasable, is still more valuable than 1a, making it the preferable limiting agent. We were again concerned about γ selectivity, as we could not predict whether higher electrophilicity of an α,β-dialkoxy aldehyde would allow allyl isomerization to remain sufficiently competitive with direct addition (see Fig. 1c for the initial analysis and Fig. 4 for the revised analysis). In fact (Fig. 2a), the transformation with ap turned out to be less regio- and diastereoselective (R,S,R-3a formed in 87:13 γ:α ratio and 72:28 d.r.). With ent-ap, regiocontrol decreased further (75:25 γ:α), but diastereoselectivity improved (98:2 d.r.), indicating this to be the matched catalyst/substrate pairing. We isolated R,R,S-3a in 70% yield (pure γ; 98:2 d.r.; X-ray structure in Fig. 2a).
We wondered whether we might take advantage of the higher electrophilicity of α,β-dialkoxy aldehyde 2. Specifically, with larger amounts of MeOH, more of the aldehyde could be converted to its derived hemiacetal, diminishing the rate of direct addition and allowing allyl shift to be more competitive. In the event, with 20 equiv. of MeOH (not 5.0 equiv.) R,R,S-3a was formed in a 95:5 γ:α ratio (compared to 75:25, previously) and with similarly high d.r. (98:2), such that it could be isolated in 93% yield (pure γ). The same applies to other electron-deficient aldehydes (3o,p, Fig. 2a). Furanose R,R,S-7 was therefore synthesized via 6 (Fig. 2b) in 73% overall yield (three steps) and >98:2 d.r. (33:67 α:β).
Another unexpected selectivity profile
Synthesis of the pyranoses (Fig. 2b) produced another surprise. Conversion of R,R,S-3a to diol R,R,S-8 was uneventfully performed (80% yield, two steps). We then treated R,R,S-8 with sodium hydride and benzyl bromide (10 mol% (n-Bu)4NI, dimethylformamide (DMF), 0.5 h, 22 °C), expecting benzyl ether formation at the primary hydroxy group. However, it was the more congested secondary alcohol—the one adjacent to the fluoro- and trifluoromethyl-substituted carbon—that reacted preferentially (Fig. 5 provides additional data and analysis). This enabled us to isolate R,R,S-9 directly in 54% yield, without needing a protection/deprotection sequence. In other words, masking of the primary alcohol and then of the secondary alcohol with a more robust protecting group, followed by selective deprotection of the primary alcohol (Fig. 1b), was not necessary. The ensuing oxidative cleavage/cyclization delivered pyranoside R,R,S-10 in 67% yield and >98:2 d.r. (>98% β). The identity of R,R,S-10 was ascertained crystallographically (Fig. 2b).
a, Preferential formation of various secondary ethers is more favoured as solvent polarity increases, suggesting a charge-separated transition state. b, Although a similar regioselectivity preference is observed with a diastereomeric substrate, regioselectivity is lower, pointing to the importance of conformational preferences. c, Formation of a tri-benzyl ether is faster, in line with higher reactivity of the more hindered site. d,e, The nature of fluoro-containing allyl substituents impacts the rate of secondary ether formation. Only when a substrate contains a trifluoromethyl- and fluoro-substituted homoallylic site is the secondary ether generated more quickly (that is, with R,R,S-3a, R,R,R-3a and not with 19 or precursors to 21 and 22). f, DFT studies provide a rationale for the unexpected reactivity of the secondary alcohols. For R,R,S-8, in the lowest-energy transition state for C–O bond formation (ts-sec-1), the proximity of the fluorine atoms elevates oxy-anion nucleophilicity. Such an interaction is not possible in ts-prim-1. g, For R,R,R-8, electrostatic attraction between the alkenyl proton and oxy-anion, facilitated by the neighbouring fluorine atom inductive effect, favours ts-sec-2′. Reactions were performed under a N2 atmosphere. Conversion and selectivity (five runs) were determined by analysis of 19F or 1H NMR spectra of unpurified mixtures (±2%). Yields are for pure products (±5%). DFT was performed at the M06-2X/6-311+G(d,p)/SMD(DMF)//M06-2X/6-31G(d)/SMD(DMF) level. See Supplementary Information part 2, section 2.2, for details. ε, dielectric constant; THF, tetrahydrofuran; DMF, dimethylformamide; Bn, benzyl; ts, transition state; sec., secondary; prim., primary.
To access R,R,R-3a (Fig. 2c), we took advantage of the stereogenic centre in aldehyde 2 as the stereocontrolling element, as originally planned. Subjection of a mixture of 2 and allylboronate 1a (98:2 Z:E) to n-butyllithium and trifluoroacetic anhydride (TFAA)40 afforded R,R,R-3a in 58% yield (pure γ) and 94:6 d.r. after purification. Conversion to R,R,R-8 was performed as before and the 1,2-diol was isolated in 78% yield. Masking of the less-hindered primary alcohol gave R,R,R-11 (>98:2 primary:secondary silyl ether), which was transformed to furanose R,R,R-12 in 84% yield and >98:2 d.r. (16:84 α:β). The oxidative procedure used to convert R,R,R-3a to 6 led to an inseparable mixture of furanose products, and the corresponding pyranose was generated. Similar to site-selective benzyl protection to obtain R,R,S-9, secondary benzyl ether/primary alcohol R,R,R-9 was isolated in 39% yield and >98:2 d.r. (after silica gel chromatography). The corresponding pyranose R,R,R-13 was obtained in 57% yield (>98:2 d.r. and 26:74 α:β). The X-ray structure of the derived para-nitrobenzoate ester endorsed the stereochemical assignment.
Organoboron or organozinc catalyst?
One unexpected observation was that, whereas the preferred enantiotopic aldehyde face was the same as initially predicted (Table 1 and Fig. 2), the major diastereomer was not the one anticipated originally. Another surprising finding was that the catalytic additions are γ-selective. To gain insight, we investigated the mechanism of the catalytic process in detail.
We first focused on allyl isomerization, required for γ-selective addition. Density functional theory (DFT; Fig. 3a) studies indicated that the energy barrier for the borotropic shift (ΔG‡ = +38.2 kcal mol−1, ts-B-1) is too steep, and even more so with a trifluoromethyl- and fluoro-substituted carbon present (ΔG‡ = 52.7 and 55.5 kcal mol−1, ts-B-2 and ts-B-3, respectively). Allylzinc isomerization, in contrast, emerged as more favourable (ts-Zn-1 and ts-Zn-2: ΔG‡ = 21.8 and 22.1 kcal mol−1, respectively). Faster isomerization of an allylzinc compared to an allylboron intermediate could be because of the longer Zn…C distances (for example, 2.27 and 2.45 Å in ts-Zn-1 compared to 1.78 and 1.86 Å in ts-B-2). There would be less lone pair–lone pair and/or steric repulsion between the catalyst framework and the allyl substituents. Whereas in ts-B-1 and ts-B-2, boron–amide coordination must be disrupted before isomerization, the same is not true for ts-Zn-1 and ts-Zn-2, both of which lead to the same Z-trisubstituted allylzinc 14 (Fig. 3b). The C–B–C angles are larger than the C–Zn–C angles (for example, 83.6° versus 64.1° for ts-B-2 and ts-Zn-1, respectively), meaning extended Zn….C distances and reduced steric pressure around the zinc centre. Enantioselectivity is therefore not impacted by the stereoisomeric purity of 1a. For example, with a 70:30 Z:E mixture of the allylboronate, S,R-3b was isolated in the same yield and regio-, diastereo- and enantioselectivity as when a 98:2 Z:E sample was used (Fig. 3b). The crystal structure of complex (ap)2Zn2 lends credence to the involvement of an aminophenol–zinc complex (Fig. 3c).
Origins of γ-, diastereo- and enantioselectivity
Kinetic investigations (Fig. 4a) show that there is first-order rate dependence on the aminophenol–zinc complex, that aldehyde concentration is inconsequential, and that the C–C-bond-formation step is probably not turnover-limiting. The orders for allylboronate (1a) and methanol are +0.3 and −0.6, respectively. In light of the above data and additional DFT-computed energy profiles, the following catalytic cycle may be proposed.
DFT studies (Supplementary Information part 2, section 2.1) indicate that aminophenol–zinc complex iii is likely in equilibrium with lower-energy multinuclear zinc aggregates, such as iii′, generated from Zn(OMe)2 (formed in situ; see Supplementary Fig. 11 for details). Reaction between iii and allyldimethoxyboronate 1a″ (ΔG‡ = 1.9 kcal mol−1), a less-hindered source of a perfluoroallyl moiety, can occur via ts-iii to give iv. The latter may be formed diastereoselectively on account of the higher steric pressure in ts-iii′, engendered by the proximity of a fluorine atom of the trifluoromethyl group and the aminophenol backbone. The stage is then set for rearrangement of iv to a lower-energy isomer (Fig. 4a). Direct addition of iv to the aldehyde would generate the minor α-addition isomer (via ts-iv, ΔG‡ = 22.5 kcal mol−1), but conversion to iv, affording allylzinc 14 (ΔG‡ = 21.8 kcal mol−1), is calculated to be more favourable. According to DFT studies, reaction of 14 with an aldehyde is faster, proceeding via ts-v (ΔG‡ = 17.5 kcal mol−1) to generate zinc–alkoxide v. Release of S,R-3b and regeneration of zinc complex iii (ΔG‡ = 9.7 kcal mol−1) completes the catalytic cycle.
Several additional points merit note. (1) Reaction via ts-iv to give α-addition isomer 4b might be slower than allylzinc isomerization due to steric pressure caused by the proximity of the trifluoromethyl to the catalyst framework (similar to ts-iii′). There is also the repulsion between the non-bonding electrons of the catalyst’s amide group and aldehyde carbonyl. The alternative ts-iv′ would be still less favourable due to propinquity of the non-bonding electrons of the amide carbonyl and the nearby fluorine atom. (2) The formation of the alternative homoallylic alcohol diastereomer via ts-v′ (ΔG‡ = 18.4 kcal mol−1) is less favoured on account of steric strain between the aldehyde substituent, trifluoromethyl group and fluorine atom. Transformation via ts-v″ (ΔG‡ = 20.4 kcal mol−1) is energetically less favourable because of electronic pressure involving the non-bonding electrons of the aldehyde and amide carbonyl groups.
Considering the rate data and the DFT findings, it may be suggested that the formation of zinc methoxide iii from catalytically inactive aggregates (for example, iii′), the generation of allylzinc iv, and its isomerization to the less congested 14 impact the overall rate. Methanol (order = −0.6) can adversely influence the reaction rate as it promotes the formation of Zn(OMe)2 and multinuclear zinc complexes, such as iii′. Methanol’s accelerating influence may be linked to its role in forming a less-hindered allylboronate (such as 1a″) and regeneration of iii. The impact of the concentration of 1a (order = +0.3) can be attributed to 1a′ and 1a″ being less hindered.
That the trifluoromethyl- and fluoro-substituted carbon is the main reason for the unusual reactivity and selectivity profiles is underscored by two control experiments (Fig. 4b). With 1b, with or without ap, homoallylic alcohol 15 was formed with similar efficiency and γ selectivity. This shows that the prenylboronate and/or the derived organozinc species are sufficiently reactive to lower the rate difference between the uncatalysed and catalytic pathways. It thus appears that a trifluoromethyl- and fluoro-substituted carbon group diminishes allylzinc nucleophilicity enough to render allyl isomerization competitive, causing improved γ selectivity. Equally revealing is the preferential formation of α-addition isomer 16 from the reaction with trifluoromethyl-substituted allylboronate 1c (Fig. 4b). Although the appreciable enantioselectivity indicates catalyst involvement, the process is α-selective, showing that in the reactions with 1a, the trifluoromethyl group as well as the fluorine substituent are needed for high γ:α ratios. In contrast, in the transformation with 1c (affording 16), direct addition to an aldehyde is faster than allyl isomerization (supported by DFT studies; Supplementary Figs. 10, 14 and 16). The greater accessibility of the zinc centre and lower electron–electron repulsion in ts-vi, compared to ts-iv′, engenders faster direct aldehyde addition compared to allylzinc rearrangement (14:86 γ:α).
The perfluoro carbon and secondary alcohol protection
The remaining question was whether the unanticipated preference for reaction at the more hindered secondary alcohol in R,R,S-8 and R,R,R-8 is because of the presence of the perfluoro stereogenic carbon (Fig. 2b,c). Preliminary studies indicated that medium polarity impacts site selectivity (Fig. 5a). Benzyl ether formation was slowest and least selective in benzene (dielectric constant (ε) = 2.27; 41% conv., 33%:33%:33% secondary:primary:bis-benzyl ether), proceeded faster and more selectively in favour of the secondary alcohol in tetrahydrofuran (THF; ε = 7.58; 61% conv., 44%:5%:51% secondary:primary:bis-benzyl ether), and was most efficient and selective in dimethylformamide (DMF, ε = 36.7; 90% conv., 62%:17%:21% secondary:primary:bis-benzyl ether). These data suggest an oxy-anion intermediate, supported by the observation that with potassium carbonate and benzyl bromide47, weakly basic conditions that are unlikely to cause much proton removal, reaction at the less-hindered primary alcohol is preferred (5.0%:20%:<2% secondary:primary:bis-benzyl ether; Fig. 5a). Another supporting example is selective silyl protection of the primary alcohol of R,R,R-8 to generate R,R,R-9 (Fig. 2c).
Further studies revealed the following. (1) In DMF and with sodium hydride, the secondary alcohol in R,R,S-8 reacts faster with other electrophiles as well (Fig. 5a). (2) The reaction affording the secondary benzyl ether derived from R,R,R-8 has a different profile (49%:14%:26% versus 62%:17%:21% secondary:primary:bis-benzyl ether; Fig. 5b) and R,R,R-9 was obtained in lower yield (39–40% versus 54% for R,R,S-9; Fig. 2b,c). (3) Protection of R,R,S-17 (Fig. 5c), with a secondary alcohol closer to the perfluoro carbon, is nearly six times faster than that of primary alcohol R,R,S-10. This underlines the impact of the perfluoro site on site selectivity, indicating that the final secondary-to-primary benzyl ether ratio also depends on the relative rates with which the initially formed benzyl ether isomers react to generate 18. (4) The transformation with 19, bearing a gem-dimethyl moiety instead of a perfluoro carbon, yielded primary benzyl ether 20 preferentially (Fig. 5d), again underscoring the impact of the perfluoro carbon centre. (5) Although reactions of homoallylic alcohols 3a, regardless of their stereoisomeric identity, were completed within 2 h, there was no conversion to 21 and 22 (Fig. 5e), in which the polyfluoro site is replaced by an allylic methyl and a gem-dimethyl moiety, respectively (THF used instead of DMF to accentuate the rate differences).
Regarding the reason for faster reaction at the more hindered secondary alcohol, it might be argued that the proximity to a trifluoromethyl- and fluoro-substituted carbon increases the acidity of the more hindered alcohol. However, inductive effects would also stabilize (lower the ground-state energy of) the oxy-anion, forcing it to be less nucleophilic (lower-energy highest-occupied molecular orbital (HOMO)). It is more likely that reaction at the secondary alcohol is faster due to enhanced electron–electron repulsion between the non-bonding electrons of the metal alkoxide and fluorine substituent (elevated ground-state energy). Increased HOMO energy translates to more favourable interaction between the oxy-anion and the anti-bonding (σ*) C–Br orbital (transition-state stabilization). DFT studies suggest that in the lowest-energy transition state for the reaction with R,R,S-8 (ts-sec-1; Fig. 5f), the trifluoromethyl group might be situated so that the oxy-anion’s nucleophilicity can be elevated. Similar interactions are not feasible in ts-prim-1, where the oxy-anion is too far from the fluorine atoms. Faster protection of the secondary alcohol in a dibenzyl ether, such as R,R,S-17 to afford R,R,S-18, probably originates from similar interactions, contributing to the secondary/primary benzyl ether ratio.
For the reaction of diastereomeric R,R,R-8 (Fig. 5g), nucleophile activation by electron–electron repulsion, as depicted in ts-sec-2, would be less favoured, probably on account of destabilizing steric repulsion between the benzyloxy and the vinyl groups (BnO–C–C–Cvinyl = 55.6° and 70.3° in ts-sec-2 and ts-sec-1, respectively). Instead, electrostatic attraction (ts-sec-2′) involving the oxy-anion and the alkenyl hydrogen are more likely to be key (ΔG‡ = 18.6 kcal mol−1; Fig. 5g). DFT studies indicate that such an interaction, while feasible for the reaction with R,R,S-8, contributes less to the reaction rate compared to ts-sec-1 (ΔG‡ = 16.7 and 19.6 kcal mol−1 for ts-sec-1 and ts-sec-1′, respectively). Former investigations show that the C–H bond of trifluoromethane or that of a CF2H moiety can serve as a hydrogen-bond donor48,49. Here, the hydrogen-bond donor ability of the alkenyl hydrogen, despite being bound to a neighbouring atom (as opposed to the carbon carrying the fluoro or the trifluoromethyl group), is enhanced by the inductive effect imposed by the trifluoromethyl group and fluorine atom in addition to hyperconjugation between the alkenyl σC–H and σ*C–F orbitals. Transformations of the saturated derivative of R,R,R-8 (obtained by hydrogenation), bearing a less effective hydrogen-bond donor, are indeed minimally selective (see Supplementary Table 2 for details).
Conclusions
We have developed diastereodivergent and enantioselective approaches for the synthesis of two furanose and two pyranose cores that contain a trifluoromethyl- and fluoro-substituted stereogenic C2. This makes it possible to access and probe the efficacy of an assortment of stereochemically defined polyfluoro oligonucleotide analogues as therapeutic agents for disease areas such as viral infections and cancer therapy. The γ-, enantio- and/or diastereoselective strategies may be extended to the synthesis of other drug candidates. For example, the corresponding catalytic additions to aldimines or ketimines may be developed for the synthesis of polyfluoro amino sugars50,51, relevant to the development of antitumour agents. The established utility of homoallylic alcohols in chemical synthesis52 suggests applications to the synthesis of therapeutic candidates that contain a polyfluoro stereogenic carbon centre.
The mechanistic principles outlined above constitute a revision of those outlined previously44, shedding light on the workings of an emerging set of chiral catalysts. We provide evidence that organic molecules bearing a trifluoromethyl- and fluoro-substituted carbon can exhibit unusual reactivity and selectivity profiles. Although additions to aldehydes involving allylzinc compounds with a trifluoromethyl group are α-selective, those containing a polyfluoro carbon are γ-selective. What is more, a trifluoromethyl- and fluoro-substituted carbon stereogenic centre and its density of non-bonding electrons, together with multiple electron-withdrawing C–F bonds, can make it preferable for C–O bond formation at a more hindered secondary hydroxy group, one that is more proximal to a trifluoromethyl- and fluoro-substituted carbon. Similar electronic alterations could also influence the interaction of derived drug candidates with biological receptors, which often contain a variety of polar moieties, imparting unique structure/activity profiles to analogues bearing this largely unexplored polyfluoro grouping.
Methods
Caution
Compound R,R,R-11 is volatile and should not be exposed to vacuum below 30 mbar. Low-boiling solvents (CH2Cl2, pentane and Et2O) were used for aqueous workup and chromatography.
Procedure for catalytic enantioselective homoallylic alcohol synthesis
In a glovebox, an oven-dried 4-ml vial containing a stir bar was charged with ap (1.6 mg, 0.0050 mmol), Zn(Ot-Bu)Et (3.1 mg, 0.013 mmol), benzaldehyde (21.4 mg, 0.200 mmol), 1a (25.4 mg, 0.100 mmol), toluene (1.0 ml) and MeOH (16.0 mg, 0.500 mmol). The vial was sealed (screw cap) and removed from the glovebox, and the mixture was allowed to stir for 1 h at 60 °C. The solution was allowed to cool to 22 °C, after which the reaction was quenched by the addition of MeOH (2.0 ml). The volatiles were removed in vacuo, leaving behind grey oil (>98% conv., based on analysis with 1H and 19F NMR spectra; 87:13 γ:α, 85:15 d.r.). Purification by silica gel chromatography (100:1 → 3:1 hexanes:Et2O) afforded S,R-3b as a colourless oil (13.8 mg, 0.0590 mmol, 59% yield, pure γ-addition isomer, >98:2 d.r., 94:6 e.r.).
Data availability
All data in support of the findings of this study are available within the Article and its Supplementary Information. X-ray crystallographic data for compounds R,R,S-3a, 3j, 5a, the p-nitrobenzoyl derivative of R,R,S-10, (ap)2Zn2, and the p-nitrobenzoyl derivative of R,R,R-13 are freely available from the Cambridge Crystallographic Data Center ((CCDC 2113846), (2113843), (2113794), (2113845), (2113842) and (2113792), respectively).
References
Zhou, Y. et al. Next generation of fluorine-containing pharmaceuticals. Compounds currently in phase II–III clinical trials of major pharmaceutical companies: new structural trends and therapeutic areas. Chem. Rev. 116, 422–518 (2016).
Meanwell, N. A. Fluorine and fluorinated motifs in the design and application of bioisosteres for drug design. J. Med. Chem. 61, 5822–5880 (2018).
Gillis, E. P., Eastman, K. J., Hill, M. D., Donnelly, D. J. & Meanwell, N. A. Applications of fluorine in medicinal chemistry. J. Med. Chem. 58, 8315–8359 (2015).
Johnson, B. M., Shu, Y.-Z., Zhuo, X. & Meanwell, N. A. Metabolic and pharmaceutical aspects of fluorinated compounds. J. Med. Chem. 63, 6315–6386 (2020).
Inoue, M., Sumii, Y. & Shibata, N. Contribution of organofluorine compounds to pharmaceuticals. ACS Omega 5, 10633–10640 (2020).
Fujiwara, T. & O′Hagan, D. Successful fluorine-containing herbicide agrochemicals. J. Fluor. Chem. 167, 16–29 (2014).
Berger, R., Resnati, G., Metrangolo, P., Weber, E. & Hulliger, J. Organic fluorine compounds: a great opportunity for enhanced materials properties. Chem. Soc. Rev. 40, 3496–3508 (2011).
Liu, P., Sharon, A. & Chu, C. K. Fluorinated nucleosides: synthesis and biological implication. J. Fluor. Chem. 129, 743–766 (2008).
Deleavy, G. F. & Damha, M. J. Designing chemically modified oligonucleotides for targeted gene silencing. Chem. Biol. 19, 937–954 (2012).
Hevey, R. The role of fluorine in glycomimetic drug design. Chem. Eur. J. 27, 2240–2253 (2020).
Kulkarni, J. A. et al. The current landscape of nucleic acid therapeutics. Nat. Nanotechnol. 16, 630–643 (2021).
Sofia, M. J. et al. Discovery of a β-d-2′-deoxy-2′-α-fluoro-2′-β-C-methyluridine nucleotide prodrug (PSI-7977) for the treatment of hepatitis C virus. J. Med. Chem. 53, 7202–7218 (2010).
Fung, A. et al. Efficiency of incorporation and chain termination determines the inhibition potency of 2′-modified nucleotide analogs against hepatitis C virus polymerase. Antimicrob. Agents Chemother. 58, 3636–3645 (2014).
Keating, G. M. Sofosbuvir: a review of its use in patients with chronic hepatitis C. Drugs 74, 1127–1146 (2014).
Appleby, T. C. et al. Structural basis for RNA replication by the hepatitis C virus polymerase. Science 347, 771–775 (2015).
Mengshetti, S. et al. Discovery of a series of 2′-α-fluoro,2′-β-bromo-ribonucleosides and their phosphoramidate prodrugs as potent pan-genotypic inhibitors of hepatitis C virus. J. Med. Chem. 62, 1859–1874 (2019).
Rillahan, C. D. et al. Global metabolic inhibitors of sialyl- and fucosyltransferases remodel the glycome. Nat. Chem. Biol. 8, 661–668 (2012).
Büll, C. et al. Targeted delivery of a sialic acid-blocking glycomimetic to cancer cells inhibits metastatic spread. ACS Nano 9, 733–745 (2015).
Baumann, A., Marchner, S., Daum, M. & Hoffmann-Röder, A. Synthesis of fluorinated Leishmania cap trisaccharides for diagnostic tool and vaccine development. Eur. J. Org. Chem. 2018, 3803–3815 (2018).
Oberbillig, T., Löwe, H. & Hoffmann-Röder, A. Synthesis of fluorinated glycosyl amino acid building blocks for MUCI cancer vaccine candidates by microreactor-assisted glycosylation. J. Flow Chem. 2, 83–86 (2012).
Klapars, A. et al. Efficient synthesis of antiviral agent uprifosbuvir enabled by new synthetic methods. Chem. Sci. 12, 9031–9036 (2021).
Beigel, J. H. et al. Remdesivir for the treatment of Covid-19 – final report. N. Engl. J. Med. 383, 1813–1826 (2020).
Jockusch, S. et al. Sofosbuvir terminated RNA is more resistant to SARS-CoV-2 proofreader than RNA terminated by remdesivir. Sci. Rep. 10, 16577 (2020).
Chien, M. et al. Nucleotide analogues as inhibitors of SARS-CoV-2 polymerase, a key drug target for COVID-19. J. Proteome Res. 19, 4690–4697 (2020).
Jeffries, B. et al. Systematic investigation of lipophilicity modulation by aliphatic fluorination motifs. J. Med. Chem. 63, 1002–1031 (2020).
Lindahl, T. Instability and decay of the primary structure of DNA. Nature 362, 709–715 (1993).
Mikkola, S., Lönnberg, T. & Lönnberg, H. Phosphodiester models for cleavage of nucleic acids. Beilstein J. Org. Chem. 14, 803–837 (2018).
Watts, J. K., Katolik, A., Viladoms, J. & Damha, M. J. Studies on the stability of 2′-fluoroarabinonucleic acid (2′F-ANA). Org. Biomol. Chem. 7, 1904–1910 (2009).
Wan, W. B. & Seth, P. P. The medicinal chemistry of therapeutic oligonucleotides. J. Med. Chem. 59, 9645–9667 (2016).
Krueger, A. C. et al. Synthesis and evaluation of 2′-dihalo ribonucleotide prodrugs with activity against hepatitis C virus. Bioorg. Med. Chem. 28, 115208 (2020).
Zhu, Y. et al. Modern approaches for asymmetric construction of carbon–fluorine quaternary stereogenic centers: synthetic challenges and pharmaceutical needs. Chem. Rev. 118, 3887–3964 (2018).
Shibata, N., Mizuta, S. & Kawai, H. Recent advances in enantioselective trifluoromethylation reactions. Tetrahedron Asymm. 19, 2633–2644 (2008).
Nie, J., Guo, H.-C., Cahard, D. & Ma, J.-A. Asymmetric construction of stereogenic carbon centers featuring a trifluoromethyl group from prochiral trifluoromethylated substrates. Chem. Rev. 111, 455–529 (2011).
Yang, X., Wu, T., Phipps, R. J. & Toste, F. D. Advances in catalytic enantioselective fluorination, mono-, di- and trifluoromethylation, and trifluoromethylthiolation reactions. Chem. Rev. 115, 826–870 (2015).
Brewitz, L. et al. Direct catalytic asymmetric Mannich-type reaction of α- and β-fluorinated amides. J. Am. Chem. Soc. 137, 15929–15939 (2015).
Brewitz, L., Kumagai, N. & Shibasaki, M. Catalytic asymmetric synthesis of 2,3,3,3-tetrafluoro-2-methyl-1-arylpropan-1-amines as useful building blocks for SAR-studies. J. Fluor. Chem. 194, 1–7 (2017).
Brewitz, L., Noda, H., Kumagai, N. & Shibasaki, M. (2R,3S)-3,4,4,4-tetrafluorovaline: a fluorinated bioisostere of isoleucine. Eur. J. Org. Chem. 2020, 1745–1752 (2020).
Meyer, S. et al. A chiral pentafluorinated isopropyl group via iodine(I)/(III) catalysis. Angew. Chem. Int. Ed. 60, 6430–6434 (2021).
Schäfer, M., Stünkel, T., Daniliuc, C. G. & Gilmour, R. Regio- and enantioselective intermolecular aminofluorination of alkenes via Iodine(I)/Iodine(III) catalysis. Angew. Chem. Int. Ed. 61, e202205508 (2022).
Chen, J. L.-Y. et al. Highly diastereo- and enantioselective allylboration of aldehydes using α-substituted allyl/crotyl pinacol boronic esters via in situ generated borinic esters. J. Am. Chem. Soc. 135, 5316–5319 (2013).
Silverio, D. L. et al. Simple organic molecules as catalysts for enantioselective synthesis of amines and alcohols. Nature 494, 216–221 (2013).
Van der Mei, F. W., Qin, C., Morrison, R. J. & Hoveyda, A. H. Practical, broadly applicable, α-selective, Z-selective, diastereoselective and enantioselective addition of allylboron compounds to mono-, di- and polyfluoroalkyl ketones. J. Am. Chem. Soc. 139, 9053–9065 (2017).
Morrison, R. J., van der Mei, F. W., Romiti, F. & Hoveyda, A. H. A catalytic approach for enantioselective synthesis of homoallylic alcohols bearing a Z-alkenyl chloride or trifluoromethyl group. A concise and protecting group-free synthesis of mycothiazole. J. Am. Chem. Soc. 142, 436–447 (2020).
Van der Mei, F. W., Miyamoto, H., Silverio, D. L. & Hoveyda, A. H. Lewis acid catalyzed borotropic shifts in the design of diastereo- and enantioselective γ-additions of allylboron moieties to aldimines. Angew. Chem. Int. Ed. 55, 4701–4706 (2016).
Paioti, P. H. S. et al. Catalytic enantioselective boryl and silyl substitution with trifluoromethyl alkenes: scope, utility and mechanistic nuances of Cu–F β-elimination. J. Am. Chem. Soc. 141, 19917–19934 (2019).
Butcher, T. W., Yang, J. L. & Hartwig, J. F. Copper-catalyzed defluorinative borylation and silylation of gem-difluoroallyl groups. Org. Lett. 22, 6805–6809 (2020).
Krishna, P. R., Reddy, V. V. R. & Srinivas, R. A new synthetic route to oxazole and pyrrole 2-deoxy-C-ribosides. Tetrahedron 63, 9871–9880 (2007).
Gu, Y., Kar, T. & Scheiner, S. Fundamental properties of the CH•••O interaction: is it a true hydrogen bond? J. Am. Chem. Soc. 121, 9411–9422 (1999).
Sessler, C. D. et al. CF2H, a hydrogen bond donor. J. Am. Chem. Soc. 139, 9325–9332 (2017).
Gruner, S. A. W., Locardi, E., Lohof, E. & Kessler, H. Carbohydrate-based mimetics in drug design: sugar amino acid and carbohydrate scaffolds. Chem. Rev. 102, 491–514 (2002).
Tacar, O., Sriamornsak, P. & Dass, C. R. Doxorubicin: an update on anticancer molecular action, toxicity and novel drug delivery systems. J. Pharmacy Pharmacol. 65, 157–170 (2012).
Boiarska, Z., Braga, T., Silvani, A. & Passarella, D. Brown allylation: application to the synthesis of natural products. Eur. J. Org. Chem. 2021, 3214–3222 (2021).
Acknowledgements
This research was supported by a grant from the National Institutes of Health (R35 GM-130395 to A.H.H. and R35 GM-128779 to P.L.). S.X. and M.J.K. were supported as LaMattina Family and Bristol-Myers Squibb Graduate Fellows, respectively. DFT calculations were performed at the Center for Research Computing at the University of Pittsburgh, the Frontera supercomputer at the Texas Advanced Computing Center, and the Extreme Science and Engineering Discovery Environment (XSEDE) supported by the National Science Foundation.
Author information
Authors and Affiliations
Contributions
S.X., J.d.P., F.R., R.J.M., K.L., S.H., M.J.K. and J.L. developed the catalytic method and carried out the applications to the synthesis of polyfluoro monosaccharides. F.R. designed the applications to polyfluoro monosaccharide syntheses. S.X., J.d.P., Y.F., B.K.M. and X.L. designed and performed the mechanistic and DFT studies. The investigations were directed by A.H.H. DFT studies were directed by P.L. A.H.H. wrote the manuscript with revisions provided by the other authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks A. Boese and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
All experimental, analytical, crystallographic and computational data. The methods by which various starting materials were prepared are presented. The 1H and 13C NMR spectra for each compound are reproduced (one per page). This document also contains the source for each reagent used, as well as an extended bibliography. There are a total of 18 tables and 20 figures.
Supplementary Data 1
Crystallographic data for compound 3j; CCDC reference 2113843.
Supplementary Data 2
Crystallographic data for compound (R,R,S)-3a; CCDC reference 2113846.
Supplementary Data 3
Crystallographic data for compound 5a; CCDC reference 2113794.
Supplementary Data 4
Crystallographic data for p-nitro-benozoate derivative of (R,R,R)-13; CCDC reference 2113792.
Supplementary Data 5
Data for X-ray structure of p-nitro-benozoate derivative of (R,R,S)-10; CCDC reference 2113845.
Supplementary Data 6
Data for X-ray structure of compound (ap)2Zn2; CCDC reference 2113842.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, S., del Pozo, J., Romiti, F. et al. Diastereo- and enantioselective synthesis of compounds with a trifluoromethyl- and fluoro-substituted carbon centre. Nat. Chem. 14, 1459–1469 (2022). https://doi.org/10.1038/s41557-022-01054-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-022-01054-4
This article is cited by
-
Site-selective chemical reactions by on-water surface sequential assembly
Nature Communications (2023)